Structural and Functional Brain Changes in Patients With Classic Trigeminal Neuralgia: A Combination of Voxel-Based Morphometry and Resting-State Functional MRI Study
- PMID: 35844235
- PMCID: PMC9277055
- DOI: 10.3389/fnins.2022.930765
Structural and Functional Brain Changes in Patients With Classic Trigeminal Neuralgia: A Combination of Voxel-Based Morphometry and Resting-State Functional MRI Study
Abstract
Objectives: Brain structural and functional abnormalities have been separately reported in patients with classic trigeminal neuralgia (CTN). However, whether and how the functional deficits are related to the structural alterations remains unclear. This study aims to investigate the anatomical and functional deficits in patients with CTN and explore their association.
Methods: A total of 34 patients with CTN and 29 healthy controls (HCs) with age- and gender-matched were recruited. All subjects underwent structural and resting-state functional magnetic resonance imaging (fMRI) scanning and neuropsychological assessments. Voxel-based morphometry (VBM) was applied to characterize the alterations of gray matter volume (GMV). The amplitude of low-frequency fluctuation (ALFF) method was used to evaluate regional intrinsic spontaneous neural activity. Further correlation analyses were performed between the structural and functional changes and neuropsychological assessments.
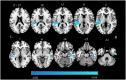
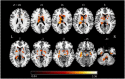
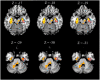
Results: Compared to the HCs, significantly reduced GMV was revealed in the right hippocampus, right fusiform gyrus (FFG), and temporal-parietal regions (the left superior/middle temporal gyrus, left operculo-insular gyrus, left inferior parietal lobule, and right inferior temporal gyrus) in patients with CTN. Increased functional activity measured by zALFF was observed mainly in the limbic system (the bilateral hippocampus and bilateral parahippocampal gyrus), bilateral FFG, basal ganglia system (the bilateral putamen, bilateral caudate, and right pallidum), left thalamus, left cerebellum, midbrain, and pons. Moreover, the right hippocampus and FFG were the overlapped regions with both functional and anatomical deficits. Furthermore, GMV in the right hippocampus was negatively correlated with pain intensity, anxiety, and depression. GMV in the right FFG was negatively correlated with illness duration. The zALFF value in the right FFG was positively correlated with anxiety.
Conclusion: Our results revealed concurrent structural and functional changes in patients with CTN, indicating that the CTN is a brain disorder with structural and functional abnormalities. Moreover, the overlapping structural and functional changes in the right hippocampus and FFG suggested that anatomical and functional changes might alter dependently in patients with CTN. These findings highlight the vital role of hippocampus and FFG in the pathophysiology of CTN.
Keywords: amplitude of low-frequency fluctuations; classic trigeminal neuralgia; neuropathic pain; resting-state functional MRI; voxel-based morphometry.
Copyright © 2022 Liu, Hou, Li, Zheng, Zhang, Cheng and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Application of amplitude of low‑frequency fluctuation to altered spontaneous neuronal activity in classical trigeminal neuralgia patients: A resting‑state functional MRI study.Mol Med Rep. 2019 Aug;20(2):1707-1715. doi: 10.3892/mmr.2019.10404. Epub 2019 Jun 20. Mol Med Rep. 2019. PMID: 31257530 Free PMC article.
-
Alterations of degree centrality and functional connectivity in classic trigeminal neuralgia.Front Neurosci. 2023 Jan 9;16:1090462. doi: 10.3389/fnins.2022.1090462. eCollection 2022. Front Neurosci. 2023. PMID: 36699513 Free PMC article.
-
Structural and functional brain abnormalities in schizophrenia: A cross-sectional study at different stages of the disease.Prog Neuropsychopharmacol Biol Psychiatry. 2018 Apr 20;83:27-32. doi: 10.1016/j.pnpbp.2017.12.017. Epub 2017 Dec 29. Prog Neuropsychopharmacol Biol Psychiatry. 2018. PMID: 29292241
-
Abnormalities of brain structure and function in cervical spondylosis: a multi-modal voxel-based meta-analysis.Front Neurosci. 2024 Jun 14;18:1415411. doi: 10.3389/fnins.2024.1415411. eCollection 2024. Front Neurosci. 2024. PMID: 38948928 Free PMC article.
-
Gray matter structural and functional brain abnormalities in Parkinson's disease: a meta-analysis of VBM and ALFF data.J Neurol. 2025 Mar 19;272(4):276. doi: 10.1007/s00415-025-12934-3. J Neurol. 2025. PMID: 40106017 Review.
Cited by
-
A multimodal meta-analysis of gray matter alterations in trigeminal neuralgia.Front Neurol. 2023 Aug 3;14:1179896. doi: 10.3389/fneur.2023.1179896. eCollection 2023. Front Neurol. 2023. PMID: 37602249 Free PMC article. Review.
-
Thalamic changes in patients with chronic facial pain.Neuroradiology. 2025 Apr;67(4):895-908. doi: 10.1007/s00234-024-03508-7. Epub 2024 Dec 7. Neuroradiology. 2025. PMID: 39644395
-
Alteration of brain network centrality in CTN patients after a single triggering pain.Front Neurosci. 2023 Feb 16;17:1109684. doi: 10.3389/fnins.2023.1109684. eCollection 2023. Front Neurosci. 2023. PMID: 36875648 Free PMC article.
-
The white matter characteristic of the genu of corpus callosum coupled with pain intensity and negative emotion scores in patients with trigeminal neuralgia: a multivariate analysis.Front Neurosci. 2024 Mar 21;18:1381085. doi: 10.3389/fnins.2024.1381085. eCollection 2024. Front Neurosci. 2024. PMID: 38576866 Free PMC article.
-
Structural brain alterations and changes in resting-state functional connectivity in patients with trigeminal neuralgia: A meta-analysis.Neuroimage Clin. 2025;46:103759. doi: 10.1016/j.nicl.2025.103759. Epub 2025 Feb 27. Neuroimage Clin. 2025. PMID: 40086208 Free PMC article. Review.
References
-
- Bennetto L., Patel N. K., Fuller G. (2007). Trigeminal neuralgia and its management. BMJ 334 201–205. 10.1136/bmj.39085.614792.BE - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

