A Strength Endurance Exercise Paradigm Mitigates Deficits in Hypoglossal-Tongue Axis Function, Strength, and Structure in a Rodent Model of Hypoglossal Motor Neuron Degeneration
- PMID: 35844238
- PMCID: PMC9279620
- DOI: 10.3389/fnins.2022.869592
A Strength Endurance Exercise Paradigm Mitigates Deficits in Hypoglossal-Tongue Axis Function, Strength, and Structure in a Rodent Model of Hypoglossal Motor Neuron Degeneration
Abstract
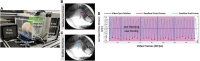
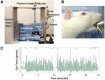
The tongue plays a crucial role in the swallowing process, and impairment can lead to dysphagia, particularly in motor neuron diseases (MNDs) resulting in hypoglossal-tongue axis degeneration (e.g., amyotrophic lateral sclerosis and progressive bulbar palsy). This study utilized our previously established inducible rodent model of dysphagia due to targeted degeneration of the hypoglossal-tongue axis. This model was created by injecting cholera toxin B conjugated to saporin (CTB-SAP) into the genioglossus muscle of the tongue base for retrograde transport to the hypoglossal (XII) nucleus via the hypoglossal nerve, which provides the sole motor control of the tongue. Our goal was to investigate the effect of high-repetition/low-resistance tongue exercise on tongue function, strength, and structure in four groups of male rats: (1) control + sham exercise (n = 13); (2) control + exercise (n = 10); (3) CTB-SAP + sham exercise (n = 13); and (4) CTB-SAP + exercise (n = 12). For each group, a custom spout with adjustable lick force requirement for fluid access was placed in the home cage overnight on days 4 and 6 post-tongue injection. For the two sham exercise groups, the lick force requirement was negligible. For the two exercise groups, the lick force requirement was set to ∼40% greater than the maximum voluntary lick force for individual rats. Following exercise exposure, we evaluated the effect on hypoglossal-tongue axis function (via videofluoroscopy), strength (via force-lickometer), and structure [via Magnetic Resonance Imaging (MRI) of the brainstem and tongue in a subset of rats]. Results showed that sham-exercised CTB-SAP rats had significant deficits in lick rate, swallow timing, and lick force. In exercised CTB-SAP rats, lick rate and lick force were preserved; however, swallow timing deficits persisted. MRI revealed corresponding degenerative changes in the hypoglossal-tongue axis that were mitigated by tongue exercise. These collective findings suggest that high-repetition/low-resistance tongue exercise in our model is a safe and effective treatment to prevent/diminish signs of hypoglossal-tongue axis degeneration. The next step is to leverage our rat model to optimize exercise dosing parameters and investigate corresponding treatment mechanisms of action for future translation to MND clinical trials.
Keywords: dysphagia; exercise; hypoglossal; motor neuron disease (MND); rodent model; tongue.
Copyright © 2022 Murphy, Thompson, Osman, Haxton, Brothers, Lee, Warncke, Smith, Keilholz, Hamad, Golzy, Bunyak, Ma, Nichols and Lever.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

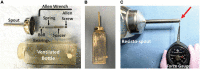





Similar articles
-
Tongue exercise ameliorates structural and functional upper airway deficits in a rodent model of hypoglossal motor neuron loss.Front Neurol. 2024 Sep 4;15:1441529. doi: 10.3389/fneur.2024.1441529. eCollection 2024. Front Neurol. 2024. PMID: 39296960 Free PMC article.
-
Hypoglossal Motor Neuron Death Via Intralingual CTB-saporin (CTB-SAP) Injections Mimic Aspects of Amyotrophic Lateral Sclerosis (ALS) Related to Dysphagia.Neuroscience. 2018 Oct 15;390:303-316. doi: 10.1016/j.neuroscience.2018.08.026. Epub 2018 Sep 1. Neuroscience. 2018. PMID: 30179644 Free PMC article.
-
Tongue and hypoglossal morphology after intralingual cholera toxin B-saporin injection.Muscle Nerve. 2021 Mar;63(3):413-420. doi: 10.1002/mus.27131. Epub 2020 Dec 15. Muscle Nerve. 2021. PMID: 33269488 Free PMC article.
-
Imaging Features of isolated hypoglossal nerve palsy.J Neuroradiol. 2020 Mar;47(2):136-150. doi: 10.1016/j.neurad.2019.04.006. Epub 2019 Apr 26. J Neuroradiol. 2020. PMID: 31034896 Review.
-
Contralateral Tongue Deviation Due to Paramedian Pontine Infarction: A Brief Review of Cortico-Hypoglossal Projections.Am J Case Rep. 2022 Jun 8;23:e936511. doi: 10.12659/AJCR.936511. Am J Case Rep. 2022. PMID: 35675222 Free PMC article. Review.
Cited by
-
Mitigating the Functional Deficit after Neurotoxic Motoneuronal Loss by an Inhibitor of Mitochondrial Fission.Int J Mol Sci. 2024 Jun 27;25(13):7059. doi: 10.3390/ijms25137059. Int J Mol Sci. 2024. PMID: 39000168 Free PMC article.
-
Tongue exercise ameliorates structural and functional upper airway deficits in a rodent model of hypoglossal motor neuron loss.Front Neurol. 2024 Sep 4;15:1441529. doi: 10.3389/fneur.2024.1441529. eCollection 2024. Front Neurol. 2024. PMID: 39296960 Free PMC article.
-
Oropharyngeal swallowing hydrodynamics of thin and mildly thick liquids in an anatomically accurate throat-epiglottis model.Sci Rep. 2024 May 25;14(1):11945. doi: 10.1038/s41598-024-60422-x. Sci Rep. 2024. PMID: 38789468 Free PMC article.
-
Radiation induced changes in profibrotic markers in the submental muscles and their correlation with tongue movement.PLoS One. 2023 Jun 23;18(6):e0287044. doi: 10.1371/journal.pone.0287044. eCollection 2023. PLoS One. 2023. PMID: 37352202 Free PMC article.
-
Task-Based Eating and Drinking Interventions in Animal Models: A Narrative Review of Functional Improvements and Neuromuscular Adaptations in Age-Related Dysphagia.Geriatrics (Basel). 2024 Oct 22;9(6):138. doi: 10.3390/geriatrics9060138. Geriatrics (Basel). 2024. PMID: 39449375 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

