First report of triterpenes pathway in Calotropis procera revealed to accumulate beta-amyrin
- PMID: 35844368
- PMCID: PMC9280243
- DOI: 10.1016/j.sjbs.2022.02.055
First report of triterpenes pathway in Calotropis procera revealed to accumulate beta-amyrin
Abstract
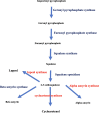
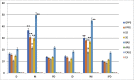
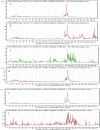
The major reports on Calotropis procera (C. procera) indicated the importance of this plant as a resource of pharmaceutically active ingredients as well as its medical advantages. β-amyrin (BA) is a significant substance in this plant and has a pharmacological effects in some frameworks, like focal and fringe sensory system, digestive and immune systems. In this study, the impact of sunlight before and after irrigation on the BA production in C. procera is studied its pathway with involved eight key enzymes. The eight enzymes' genes were characterized and successfully submitted to NCBI; AAS (acc.no. KU997645) for α-amyrin synthase, BAS (acc.no. MW976955) for β-amyrin synthase, SE (acc.no. MW976956) for squalene epoxidase, SS (acc.no. MW976957) for squalene synthase, GPPS, (acc.no. MW976958) for geranyl pyrophosphate synthase, FPPS (acc.no. MW976959) for farnasyl pyrophosphate synthase, CAS1, (acc.no. MZ00598) for cycloartenol synthase1 and LS (acc.no. MZ005982) for lupeol synthase. qRT-PCR analysis revealed high expression levels of GPPS, FPPS, SS, SE, and BAS genes at all times specially midday. Otherwise, CAS1, LS and BAS expression levels were very low at all daylight periods. The UPLC β-amyrin data are in accordance with qRT-PCR results. This indicates that triterpenes biosynthetic pathway in C. procera is going to β-amyrin accumulation with the highest level at midday.
Keywords: Beta-amyrin biosynthesis; Calotropis procera; Hydration; Light.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Calotropis procera accumulates Uzarigenin and Calotropagenin in response to environmental lighting and drought.Saudi J Biol Sci. 2023 Apr;30(4):103622. doi: 10.1016/j.sjbs.2023.103622. Epub 2023 Mar 9. Saudi J Biol Sci. 2023. PMID: 36950364 Free PMC article.
-
Pathway engineering for the production of β-amyrin and cycloartenol in Escherichia coli-a method to biosynthesize plant-derived triterpene skeletons in E. coli.Appl Microbiol Biotechnol. 2017 Sep;101(17):6615-6625. doi: 10.1007/s00253-017-8409-z. Epub 2017 Jul 15. Appl Microbiol Biotechnol. 2017. PMID: 28710558
-
Control of β-sitosterol biosynthesis under light and watering in desert plant Calotropis procera.Steroids. 2019 Jan;141:1-8. doi: 10.1016/j.steroids.2018.11.003. Epub 2018 Nov 7. Steroids. 2019. PMID: 30414421
-
Key enzymes of triterpenoid saponin biosynthesis and the induction of their activities and gene expressions in plants.Nat Prod Commun. 2010 Jul;5(7):1147-58. Nat Prod Commun. 2010. PMID: 20734961 Review.
-
β-Amyrin biosynthesis: catalytic mechanism and substrate recognition.Org Biomol Chem. 2017 Apr 5;15(14):2869-2891. doi: 10.1039/c7ob00238f. Org Biomol Chem. 2017. PMID: 28294269 Review.
Cited by
-
Calotropis procera accumulates Uzarigenin and Calotropagenin in response to environmental lighting and drought.Saudi J Biol Sci. 2023 Apr;30(4):103622. doi: 10.1016/j.sjbs.2023.103622. Epub 2023 Mar 9. Saudi J Biol Sci. 2023. PMID: 36950364 Free PMC article.
References
-
- Aragão G.F., Carneiro L.M., Junior A.P., Vieira L.C., Bandeira P.N., Lemos T.L., Viana G.S. A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacol Biochem Behav. 2006;85(4):827–834. doi: 10.1016/j.pbb.2006.11.019. - DOI - PubMed
-
- Babalola I.T., Shode F.O. Ubiquitous ursolic acid: a potential pentacyclic triterpene natural product. J. Pharmacogn. Phytochem. 2013;2(2):214–222.
-
- Bandeira P.N., Lemos T.L.G., Costa S.M.O., Santos H.S. Obtenção de derivados da mistura triterpenoídica α- e β-amirina. Rev. Bras. Farmacogn. 2007;17:204–208.
-
- Barros A.B., Moura A.F., Silva D.A., Oliveira T.M., Barreto F.S., Ribeiro W.L.C., Alves A.P.N.N., Araújo A.J., FILHO MORAES, B ILES, J.V.R. MEDEIROS, J.D.B MARINHO-FILHO. Evaluation of antitumor potential of cashew gum extracted from Anacardium occidentale Linn. International Journal of Biological Macromolecules. 2020;154:319–328. - PubMed
LinkOut - more resources
Full Text Sources

