Polyamines mitigate the destructive impacts of salinity stress by enhancing photosynthetic capacity, antioxidant defense system and upregulation of calvin cycle-related genes in rapeseed (Brassica napus L.)
- PMID: 35844395
- PMCID: PMC9280241
- DOI: 10.1016/j.sjbs.2022.02.053
Polyamines mitigate the destructive impacts of salinity stress by enhancing photosynthetic capacity, antioxidant defense system and upregulation of calvin cycle-related genes in rapeseed (Brassica napus L.)
Abstract
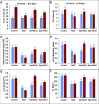
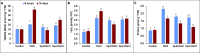
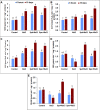
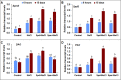
Salinity is widespread environmental stress that poses great obstacles to rapeseed development and growth. Polyamines are key plant growth regulators that play a pivotal role in regulating salt tolerance. Rapeseed (Brassica napus L.) seedlings were treated by spermine (Spm) and spermidine (Spd) versus untreated control under salt stress conditions. It was detected that the Spd-treated plants had significantly elevated chlorophyll and proline content and maintained higher photosystem II (PSII) activity than those treated with Spm as well as untreated control under salt-stressed conditions. Similarly, Spd alleviated the devastating effects of NaCl stress on CO2 assimilation and significantly elevated Rubisco activity (ribulose 1,5-bisphosphate carboxylase/oxygenase). The application of Spd also enhanced the activities of different antioxidant enzymes under NaCl stress. It modulated their respective transcription levels, including ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and dehydroascorbate reductase (DHAR). In addition, exogenously sprayed Spd enhanced the polyamine pathway as observed by upregulated transcription of polyamine oxidase (PAO) and diamine oxidase (DAO). The Spd application enhanced expressions of Calvin cycle enzyme related genes such as Rubisco small subunit, Rubisco large subunit, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 3-phosphoglyceric acid kinase (PGK), triose-3-phosphate isomerase (TPI), fructose-1,6-bisphosphate aldolase (FBA), sedoheptulose-1,7-bisphosphatase (SBPase), and fructose-1,6-bisphosphate phosphatase (FBPase). Consequently, this study demonstrates that exogenous application of Spd has a valuable role in regulating antioxidant enzyme activity, polyamine pathway, and Calvin cycle enzyme-related genes to alleviate salt stress damage in the plants.
Keywords: Gene expression; Photosynthesis; Rapeseed; Salinity stress; Spermidine; Spermine.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Effects of exogenous spermidine on photosynthetic capacity and expression of Calvin cycle genes in salt-stressed cucumber seedlings.J Plant Res. 2014 Nov;127(6):763-73. doi: 10.1007/s10265-014-0653-z. Epub 2014 Jul 29. J Plant Res. 2014. PMID: 25069716
-
Enhancing antioxidant systems by exogenous spermine and spermidine in wheat (Triticum aestivum) seedlings exposed to salt stress.Funct Plant Biol. 2018 Jun;45(7):745-759. doi: 10.1071/FP17127. Funct Plant Biol. 2018. PMID: 32291049
-
Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system.Gene. 2014 Mar 1;537(1):70-8. doi: 10.1016/j.gene.2013.12.021. Epub 2013 Dec 21. Gene. 2014. PMID: 24365592
-
Melatonin Improves Salt Tolerance in Tomato Seedlings by Enhancing Photosystem II Functionality and Calvin Cycle Activity.Plants (Basel). 2025 Jun 11;14(12):1785. doi: 10.3390/plants14121785. Plants (Basel). 2025. PMID: 40573778 Free PMC article.
-
Regulation of Calvin-Benson cycle enzymes under high temperature stress.aBIOTECH. 2022 Jan 24;3(1):65-77. doi: 10.1007/s42994-022-00068-3. eCollection 2022 Mar. aBIOTECH. 2022. PMID: 36311539 Free PMC article. Review.
Cited by
-
6-deoxy-6-amino chitosan: a preventative treatment in the tomato/Botrytis cinerea pathosystem.Front Plant Sci. 2023 Oct 10;14:1282050. doi: 10.3389/fpls.2023.1282050. eCollection 2023. Front Plant Sci. 2023. PMID: 37881612 Free PMC article.
-
Putrescine mitigates NaCl-induced stress by modulating gene expression, antioxidants, and ethylene level in tomato.Plant Signal Behav. 2025 Dec;20(1):2515431. doi: 10.1080/15592324.2025.2515431. Epub 2025 Jun 16. Plant Signal Behav. 2025. PMID: 40524453 Free PMC article.
-
Exploring Salinity Tolerance Mechanisms in Diverse Wheat Genotypes Using Physiological, Anatomical, Agronomic and Gene Expression Analyses.Plants (Basel). 2023 Sep 20;12(18):3330. doi: 10.3390/plants12183330. Plants (Basel). 2023. PMID: 37765494 Free PMC article.
-
Roles of S-Adenosylmethionine and Its Derivatives in Salt Tolerance of Cotton.Int J Mol Sci. 2023 May 30;24(11):9517. doi: 10.3390/ijms24119517. Int J Mol Sci. 2023. PMID: 37298464 Free PMC article. Review.
-
Applying microbial biostimulants and drought-tolerant genotypes to enhance barley growth and yield under drought stress.Front Plant Sci. 2025 Jan 7;15:1494987. doi: 10.3389/fpls.2024.1494987. eCollection 2024. Front Plant Sci. 2025. PMID: 39840355 Free PMC article. Review.
References
-
- Alexieva V., Sergiev I., Mapelli S., Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell Environ. 2001;24:1337–1344.
-
- Alscher R.G., Erturk N., Heath L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002;53:1331–1341. - PubMed
-
- Baker N.R., Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J. Exp. Bot. 2004;55:1607–1621. - PubMed
-
- Bela K., Riyazuddin R., Horváth E., Hurton Á., Gallé Á., Takács Z., Zsigmond L., Szabados L., Tari I., Csiszár J. Comprehensive analysis of antioxidant mechanisms in Arabidopsis glutathione peroxidase-like mutants under salt-and osmotic stress reveals organ-specific significance of the AtGPXL’s activities. Environ. Exp. Bot. 2018;150:127–140.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

