Synergies of Extracellular Vesicles and Microchimerism in Promoting Immunotolerance During Pregnancy
- PMID: 35844513
- PMCID: PMC9285877
- DOI: 10.3389/fimmu.2022.837281
Synergies of Extracellular Vesicles and Microchimerism in Promoting Immunotolerance During Pregnancy
Abstract
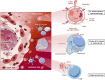
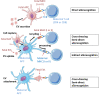
The concept of biological identity has been traditionally a central issue in immunology. The assumption that entities foreign to a specific organism should be rejected by its immune system, while self-entities do not trigger an immune response is challenged by the expanded immunotolerance observed in pregnancy. To explain this "immunological paradox", as it was first called by Sir Peter Medawar, several mechanisms have been described in the last decades. Among them, the intentional transfer and retention of small amounts of cells between a mother and her child have gained back attention. These microchimeric cells contribute to expanding allotolerance in both organisms and enhancing genetic fitness, but they could also provoke aberrant alloimmune activation. Understanding the mechanisms used by microchimeric cells to exert their function in pregnancy has proven to be challenging as per definition they are extremely rare. Profiting from studies in the field of transplantation and cancer research, a synergistic effect of microchimerism and cellular communication based on the secretion of extracellular vesicles (EVs) has begun to be unveiled. EVs are already known to play a pivotal role in feto-maternal tolerance by transferring cargo from fetal to maternal immune cells to reshape their function. A further aspect of EVs is their function in antigen presentation either directly or on the surface of recipient cells. Here, we review the current understanding of microchimerism in the feto-maternal tolerance during human pregnancy and the potential role of EVs in mediating the allorecognition and tropism of microchimeric cells.
Keywords: allorecognition; cross-dressing; extracellular vesicles (EV); immunotolerance; microchimerism; pregnancy.
Copyright © 2022 Murrieta-Coxca, Fuentes-Zacarias, Ospina-Prieto, Markert and Morales-Prieto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The 'communicatome' of pregnancy: spotlight on cellular and extravesicular chimerism.EMBO Mol Med. 2024 Apr;16(4):700-714. doi: 10.1038/s44321-024-00045-x. Epub 2024 Mar 11. EMBO Mol Med. 2024. PMID: 38467841 Free PMC article. Review.
-
Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance.Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):1099-1104. doi: 10.1073/pnas.1618364114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096390 Free PMC article.
-
Are fetal microchimerism and circulating fetal extracellular vesicles important links between spontaneous preterm delivery and maternal cardiovascular disease risk?Bioessays. 2024 Apr;46(4):e2300170. doi: 10.1002/bies.202300170. Epub 2024 Feb 15. Bioessays. 2024. PMID: 38359068
-
Extracellular vesicles in allograft rejection and tolerance.Cell Immunol. 2020 Mar;349:104063. doi: 10.1016/j.cellimm.2020.104063. Epub 2020 Feb 8. Cell Immunol. 2020. PMID: 32087929 Free PMC article. Review.
-
Deciphering the Role of Maternal Microchimerism in Offspring Autoimmunity: A Narrative Review.Medicina (Kaunas). 2024 Sep 5;60(9):1457. doi: 10.3390/medicina60091457. Medicina (Kaunas). 2024. PMID: 39336498 Free PMC article. Review.
Cited by
-
HLA-G and Recurrent Pregnancy Loss.Int J Mol Sci. 2023 Jan 29;24(3):2557. doi: 10.3390/ijms24032557. Int J Mol Sci. 2023. PMID: 36768880 Free PMC article. Review.
-
Platelet and mitochondrial RNA is decreased in plasma-derived extracellular vesicles in women with preeclampsia-an exploratory study.BMC Med. 2023 Nov 23;21(1):458. doi: 10.1186/s12916-023-03178-x. BMC Med. 2023. PMID: 37996819 Free PMC article.
-
The when, what, and where of naturally-acquired microchimerism.Semin Immunopathol. 2025 Mar 11;47(1):20. doi: 10.1007/s00281-024-01029-2. Semin Immunopathol. 2025. PMID: 40067465 Review.
-
Characterization of fetal microchimeric immune cells in mouse maternal hearts during physiologic and pathologic pregnancies.Front Cell Dev Biol. 2023 Sep 22;11:1256945. doi: 10.3389/fcell.2023.1256945. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37808080 Free PMC article.
-
Expression of placental glycans and its role in regulating peripheral blood NK cells during preeclampsia: a perspective.Front Endocrinol (Lausanne). 2023 May 3;14:1087845. doi: 10.3389/fendo.2023.1087845. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37206444 Free PMC article. Review.
References
-
- Medawar PB. Peter Medawar – Nobel Lecture. NobelPrize.org. Nobel Prize Outreach AB 2022 (1960). Available at: https://www.nobelprize.org/prizes/medicine/1960/medawar/lecture/ (Accessed 2022).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

