Gasdermins: New Therapeutic Targets in Host Defense, Inflammatory Diseases, and Cancer
- PMID: 35844522
- PMCID: PMC9285118
- DOI: 10.3389/fimmu.2022.898298
Gasdermins: New Therapeutic Targets in Host Defense, Inflammatory Diseases, and Cancer
Abstract
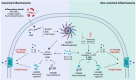
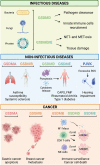
Gasdermins (GSDMs) are a class of pore-forming proteins related to pyroptosis, a programmed cell death pathway that is induced by a range of inflammatory stimuli. Small-scale GSDM activation and pore formation allow the passive release of cytokines, such as IL-1β and IL-18, and alarmins, but, whenever numerous GSDM pores are assembled, osmotic lysis and cell death occur. Such GSDM-mediated pyroptosis promotes pathogen clearance and can help restore homeostasis, but recent studies have revealed that dysregulated pyroptosis is at the root of many inflammation-mediated disease conditions. Moreover, new homeostatic functions for gasdermins are beginning to be revealed. Here, we review the newly discovered mechanisms of GSDM activation and their prominent roles in host defense and human diseases associated with chronic inflammation. We also highlight the potential of targeting GSDMs as a new therapeutic approach to combat chronic inflammatory diseases and cancer and how we might overcome the current obstacles to realize this potential.
Keywords: cancer; gasdermins; host defense; inflammasome; pyroptosis; therapeutics.
Copyright © 2022 Magnani, Colantuoni and Mortellaro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

