Key Factors for Thymic Function and Development
- PMID: 35844535
- PMCID: PMC9280625
- DOI: 10.3389/fimmu.2022.926516
Key Factors for Thymic Function and Development
Abstract
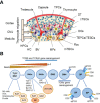
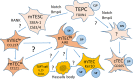
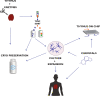
The thymus is the organ responsible for T cell development and the formation of the adaptive immunity function. Its multicellular environment consists mainly of the different stromal cells and maturing T lymphocytes. Thymus-specific progenitors of epithelial, mesenchymal, and lymphoid cells with stem cell properties represent only minor populations. The thymic stromal structure predominantly determines the function of the thymus. The stromal components, mostly epithelial and mesenchymal cells, form this specialized area. They support the consistent developmental program of functionally distinct conventional T cell subpopulations. These include the MHC restricted single positive CD4+ CD8- and CD4- CD8+ cells, regulatory T lymphocytes (Foxp3+), innate natural killer T cells (iNKT), and γδT cells. Several physiological causes comprising stress and aging and medical treatments such as thymectomy and chemo/radiotherapy can harm the thymus function. The present review summarizes our knowledge of the development and function of the thymus with a focus on thymic epithelial cells as well as other stromal components and the signaling and transcriptional pathways underlying the thymic cell interaction. These critical thymus components are significant for T cell differentiation and restoring the thymic function after damage to reach the therapeutic benefits.
Keywords: T cells; intrathymic regulators; thymic epithelial cells (TEC); thymic microenvironment; thymic stem cells; thymus; thymus regeneration.
Copyright © 2022 Shichkin and Antica.
Conflict of interest statement
Author VS was employed by company OmniFarma. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Muсoz JJ, Zapata AG. “Thymus Ontogeny and Development”. In: Passos GA, editor. Thymus Transcriptome and Cell Biology. Springer Nature Switzerland AG, Cham: Springer Nature Switzerland AG; (2019). p. 19–34. doi: 10.1007/978-3-030-12040-5_2 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

