Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies
- PMID: 35844552
- PMCID: PMC9279668
- DOI: 10.3389/fimmu.2022.929895
Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies
Abstract
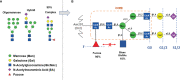
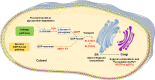
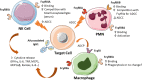
The presence of fucose on IgG1 Asn-297 N-linked glycan is the modification of the human IgG1 Fc structure with the most significant impact on FcɣRIII affinity. It also significantly enhances the efficacy of antibody dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells in vitro, induced by IgG1 therapeutic monoclonal antibodies (mAbs). The effect of afucosylation on ADCC or antibody dependent phagocytosis (ADCP) mediated by macrophages or polymorphonuclear neutrophils (PMN) is less clear. Evidence for enhanced efficacy of afucosylated therapeutic mAbs in vivo has also been reported. This has led to the development of several therapeutic antibodies with low Fc core fucose to treat cancer and inflammatory diseases, seven of which have already been approved for clinical use. More recently, the regulation of IgG Fc core fucosylation has been shown to take place naturally during the B-cell immune response: A decrease in α-1,6 fucose has been observed in polyclonal, antigen-specific IgG1 antibodies which are generated during alloimmunization of pregnant women by fetal erythrocyte or platelet antigens and following infection by some enveloped viruses and parasites. Low IgG1 Fc core fucose on antigen-specific polyclonal IgG1 has been linked to disease severity in several cases, such as SARS-CoV 2 and Dengue virus infection and during alloimmunization, highlighting the in vivo significance of this phenomenon. This review aims to summarize the current knowledge about human IgG1 Fc core fucosylation and its regulation and function in vivo, in the context of both therapeutic antibodies and the natural immune response. The parallels in these two areas are informative about the mechanisms and in vivo effects of Fc core fucosylation, and may allow to further exploit the desired properties of this modification in different clinical contexts.
Keywords: ADCC; IgG; N-glycan; NK cells; fucosylation; humoral response; therapeutic antibodies; virus.
Copyright © 2022 Golay, Andrea and Cattaneo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Pezer M, Pezer M. Immunoglobulin G Glycosylation in Diseases. In: Antibody Glycosylation, vol. 112. . Cham: Springer International Publishing; (2021). doi: 10.1007/978-3-030-76912-3_13 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

