Commonalities of Mycobacterium tuberculosis Transcriptomes in Response to Defined Persisting Macrophage Stresses
- PMID: 35844560
- PMCID: PMC9283954
- DOI: 10.3389/fimmu.2022.909904
Commonalities of Mycobacterium tuberculosis Transcriptomes in Response to Defined Persisting Macrophage Stresses
Abstract
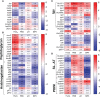
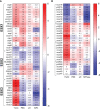
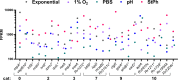
As the goal of a bacterium is to become bacteria, evolution has imposed continued selections for gene expression. The intracellular pathogen Mycobacterium tuberculosis, the causative agent of tuberculosis, has adopted a fine-tuned response to survive its host's methods to aggressively eradicate invaders. The development of microarrays and later RNA sequencing has led to a better understanding of biological processes controlling the relationship between host and pathogens. In this study, RNA-seq was performed to detail the transcriptomes of M. tuberculosis grown in various conditions related to stresses endured by M. tuberculosis during host infection and to delineate a general stress response incurring during persisting macrophage stresses. M. tuberculosis was subjected to long-term growth, nutrient starvation, hypoxic and acidic environments. The commonalities between these stresses point to M. tuberculosis maneuvering to exploit propionate metabolism for lipid synthesis or to withstand propionate toxicity whilst in the intracellular environment. While nearly all stresses led to a general shutdown of most biological processes, up-regulation of pathways involved in the synthesis of amino acids, cofactors, and lipids were observed only in hypoxic M. tuberculosis. This data reveals genes and gene cohorts that are specifically or exclusively induced during all of these persisting stresses. Such knowledge could be used to design novel drug targets or to define possible M. tuberculosis vulnerabilities for vaccine development. Furthermore, the disruption of specific functions from this gene set will enhance our understanding of the evolutionary forces that have caused the tubercle bacillus to be a highly successful pathogen.
Keywords: RNA-seq; dormancy; hypoxia; pH; starvation; tuberculosis.
Copyright © 2022 Vilchèze, Yan, Casey, Hingley-Wilson, Ettwiller and Jacobs.
Conflict of interest statement
BY and LE are employees of New England Biolabs, a US company that sells research reagents (such as RNA reagents and library preparation kits) to the scientific community. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
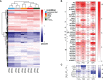
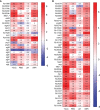
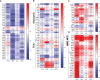
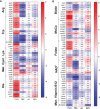
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

