Immunogenicity of SARS-CoV-2 Trimeric Spike Protein Associated to Poly(I:C) Plus Alum
- PMID: 35844561
- PMCID: PMC9281395
- DOI: 10.3389/fimmu.2022.884760
Immunogenicity of SARS-CoV-2 Trimeric Spike Protein Associated to Poly(I:C) Plus Alum
Abstract
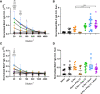
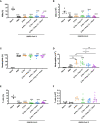
The SARS-CoV-2 pandemic has had a social and economic impact worldwide, and vaccination is an efficient strategy for diminishing those damages. New adjuvant formulations are required for the high vaccine demands, especially adjuvant formulations that induce a Th1 phenotype. Herein we assess a vaccination strategy using a combination of Alum and polyinosinic:polycytidylic acid [Poly(I:C)] adjuvants plus the SARS-CoV-2 spike protein in a prefusion trimeric conformation by an intradermal (ID) route. We found high levels of IgG anti-spike antibodies in the serum by enzyme linked immunosorbent assay (ELISA) and high neutralizing titers against SARS-CoV-2 in vitro by neutralization assay, after two or three immunizations. By evaluating the production of IgG subtypes, as expected, we found that formulations containing Poly(I:C) induced IgG2a whereas Alum did not. The combination of these two adjuvants induced high levels of both IgG1 and IgG2a. In addition, cellular immune responses of CD4+ and CD8+ T cells producing interferon-gamma were equivalent, demonstrating that the Alum + Poly(I:C) combination supported a Th1 profile. Based on the high neutralizing titers, we evaluated B cells in the germinal centers, which are specific for receptor-binding domain (RBD) and spike, and observed that more positive B cells were induced upon the Alum + Poly(I:C) combination. Moreover, these B cells produced antibodies against both RBD and non-RBD sites. We also studied the impact of this vaccination preparation [spike protein with Alum + Poly(I:C)] in the lungs of mice challenged with inactivated SARS-CoV-2 virus. We found a production of IgG, but not IgA, and a reduction in neutrophil recruitment in the bronchoalveolar lavage fluid (BALF) of mice, suggesting that our immunization scheme reduced lung inflammation. Altogether, our data suggest that Alum and Poly(I:C) together is a possible adjuvant combination for vaccines against SARS-CoV-2 by the intradermal route.
Keywords: SARS-CoV-2; adjuvants; alum; intradermal route; poly (I:C); spike protein; vaccine.
Copyright © 2022 dos-Santos, Firmino-Cruz, da Fonseca-Martins, Oliveira-Maciel, Perez, Roncaglia-Pereira, Dumard, Guedes-da-Silva, Santos, Leandro, Ferreira, Guimarães-Pinto, Conde, Rodrigues, Silva, Alvim, Lima, Marsili, Abreu, Ferreira Jr., Mohana Borges, Tanuri, Souza, Rossi-Bergmann, Vale, Silva, de Oliveira, Filardy, Gomes and de Matos Guedes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Tebas P, Kimberly AK, Ami P, Joel NM, Matthew PM, Albert JS, et al. Intradermal Syncon® Ebola GP DNA Vaccine Is Temperature Stable And Safely Demonstrates Cellular And Humoral Immunogenicity Advantages In Healthy Volunteers. J Of Infect Dis (2019) 220(3):400–10. doi: 10.1093/infdis/jiz132 - DOI - PubMed
-
- Frey SE, Anna W, Srilatha E, Lisa AJ, Jack TS, Hana ES, et al. Comparison Of Lyophilized Versus Liquid Modified Vaccinia Ankara (Mva) Formulations and Subcutaneous Versus Intradermal Routes Of Administration In Healthy Vaccinia-Naïve Subjects”. Vaccine (2015) 33(39):5225–34. doi: 10.1016/j.vaccine.2015.06.075 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

