Naïve Primary Mouse CD8+ T Cells Retain In Vivo Immune Responsiveness After Electroporation-Based CRISPR/Cas9 Genetic Engineering
- PMID: 35844563
- PMCID: PMC9280190
- DOI: 10.3389/fimmu.2022.777113
Naïve Primary Mouse CD8+ T Cells Retain In Vivo Immune Responsiveness After Electroporation-Based CRISPR/Cas9 Genetic Engineering
Abstract
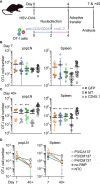
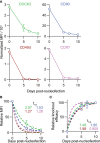
CRISPR/Cas9 technology has revolutionized genetic engineering of primary cells. Although its use is gaining momentum in studies on CD8+ T cell biology, it remains elusive to what extent CRISPR/Cas9 affects in vivo function of CD8+ T cells. Here, we optimized nucleofection-based CRISPR/Cas9 genetic engineering of naïve and in vitro-activated primary mouse CD8+ T cells and tested their in vivo immune responses. Nucleofection of naïve CD8+ T cells preserved their in vivo antiviral immune responsiveness to an extent that is indistinguishable from non-nucleofected cells, whereas nucleofection of in vitro-activated CD8+ T cells led to slightly impaired expansion/survival at early time point after adoptive transfer and more pronounced contraction. Of note, different target proteins displayed distinct decay rates after gene editing. This is in stark contrast to a comparable period of time required to complete gene inactivation. Thus, for optimal experimental design, it is crucial to determine the kinetics of the loss of target gene product to adapt incubation period after gene editing. In sum, nucleofection-based CRISPR/Cas9 genome editing achieves efficient and rapid generation of mutant CD8+ T cells without imposing detrimental constraints on their in vivo functions.
Keywords: CD8+ T cell genetic engineering; CRISPR/Cas9; antiviral immunity; gene inactivation; nucleofection; primary CD8+ T cell; target protein depletion.
Copyright © 2022 Pfenninger, Yerly and Abe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

