Case Report: Complete Remission of a Patient With Metastatic Gastric Cancer Treated With Nivolumab Combined With Chemotherapy After Palliative Surgery
- PMID: 35844567
- PMCID: PMC9278084
- DOI: 10.3389/fimmu.2022.908558
Case Report: Complete Remission of a Patient With Metastatic Gastric Cancer Treated With Nivolumab Combined With Chemotherapy After Palliative Surgery
Abstract
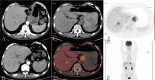
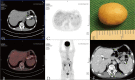
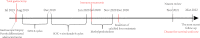
Metastatic advanced gastric cancer, for which treatment strategies are extremely limited, has a poor prognosis. Complete remission is rare. Patients usually lose the opportunity of therapeutic surgery because the lesions cannot be completely removed, although it can greatly prolong their survival time. Palliative surgery usually suggests bad outcomes. In recent years, the immune checkpoint inhibitor (ICI) nivolumab has shown significant efficacy in the treatment of advanced gastric cancer. However, its applicable conditions and optimal withdrawal time remain controversial owing to its low response rate and high incidence of immune-related adverse events. Herein, we introduce a 66-year-old male patient with advanced gastric cancer with multiple liver metastases who underwent laparoscopic total gastrectomy for acute gastric bleeding. The patient received eight cycles of S-1 plus oxaliplatin (SOX) and switched to eight cycles of SOX plus nivolumab combined regimen in a stable state, later achieving complete remission. There was no recurrence for 32 months after the surgery. This is the first reported case of gastric cancer with multiple liver metastases with long-term complete remission with nivolumab treatment after palliative surgery. The potential mechanism of complete remission was discussed through clinical, genomic, and immune characteristics. The patient had a history of psoriasis and was positive for programmed death ligand 1 (PD-L1), and the interaction of TP53 mutation and HER-2 (-) gene may be associated with complete remission.
Keywords: advanced gastric cancer; case report; complete remission; immune checkpoint inhibitor; nivolumab.
Copyright © 2022 Dai, Rao, Zhang, Qiu, Wu, Lin, Li, Li, Cai and Han.
Conflict of interest statement
Author SL was employed by company Burning Rock Biotech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kim SG, Seo HS, Lee HH, Song KY, Park CH. Comparison of the Differences in Survival Rates Between the 7th and 8th Editions of the AJCC TNM Staging System for Gastric Adenocarcinoma: A Single-Institution Study of 5,507 Patients in Korea. J Gastric Canc (2017) 17:212–9. doi: 10.5230/jgc.2017.17.e23 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

