Claudin-1 Mediated Tight Junction Dysfunction as a Contributor to Atopic March
- PMID: 35844593
- PMCID: PMC9277052
- DOI: 10.3389/fimmu.2022.927465
Claudin-1 Mediated Tight Junction Dysfunction as a Contributor to Atopic March
Abstract
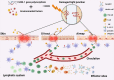
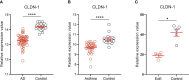
Atopic march refers to the phenomenon wherein the occurrence of asthma and food allergy tends to increase after atopic dermatitis. The mechanism underlying the progression of allergic inflammation from the skin to gastrointestinal (GI) tract and airways has still remained elusive. Impaired skin barrier was proposed as a risk factor for allergic sensitization. Claudin-1 protein forms tight junctions and is highly expressed in the epithelium of the skin, airways, and GI tract, thus, the downregulation of claudin-1 expression level caused by CLDN-1 gene polymorphism can mediate common dysregulation of epithelial barrier function in these organs, potentially leading to allergic sensitization at various sites. Importantly, in patients with atopic dermatitis, asthma, and food allergy, claudin-1 expression level was significantly downregulated in the skin, bronchial and intestinal epithelium, respectively. Knockdown of claudin-1 expression level in mouse models of atopic dermatitis and allergic asthma exacerbated allergic inflammation, proving that downregulation of claudin-1 expression level contributes to the pathogenesis of allergic diseases. Therefore, we hypothesized that the tight junction dysfunction mediated by downregulation of claudin-1 expression level contributes to atopic march. Further validation with clinical data from patients with atopic march or mouse models of atopic march is needed. If this hypothesis can be fully confirmed, impaired claudin-1 expression level may be a risk factor and likely a diagnostic marker for atopic march. Claudin-1 may serve as a valuable target to slowdown or block the progression of atopic march.
Keywords: asthma; atopic dermatitis; atopic march; claudin-1; epithelial barrier; food allergy.
Copyright © 2022 Xia, Cao, Zheng and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis.Am J Pathol. 2015 Oct;185(10):2777-89. doi: 10.1016/j.ajpath.2015.06.021. Epub 2015 Aug 28. Am J Pathol. 2015. PMID: 26319240
-
Scratching damages tight junctions through the Akt-claudin 1 axis in atopic dermatitis.Clin Exp Dermatol. 2021 Jan;46(1):74-81. doi: 10.1111/ced.14380. Epub 2020 Sep 5. Clin Exp Dermatol. 2021. PMID: 32668051
-
Bortezomib, a proteasome inhibitor, alleviates atopic dermatitis by increasing claudin 1 protein expression.Biochem Biophys Res Commun. 2017 Nov 4;493(1):744-750. doi: 10.1016/j.bbrc.2017.08.120. Epub 2017 Aug 30. Biochem Biophys Res Commun. 2017. PMID: 28859979
-
Epithelial barrier dysfunctions in atopic dermatitis: a skin-gut-lung model linking microbiome alteration and immune dysregulation.Br J Dermatol. 2018 Sep;179(3):570-581. doi: 10.1111/bjd.16734. Epub 2018 Jul 11. Br J Dermatol. 2018. PMID: 29761483 Review.
-
Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation.Ann N Y Acad Sci. 2017 Jun;1397(1):66-79. doi: 10.1111/nyas.13360. Epub 2017 May 10. Ann N Y Acad Sci. 2017. PMID: 28493289 Free PMC article. Review.
Cited by
-
Epithelial Barrier Theory: The Role of Exposome, Microbiome, and Barrier Function in Allergic Diseases.Allergy Asthma Immunol Res. 2023 Nov;15(6):705-724. doi: 10.4168/aair.2023.15.6.705. Allergy Asthma Immunol Res. 2023. PMID: 37957791 Free PMC article. Review.
-
Atopic dermatitis and food allergy: More than sensitization.Mucosal Immunol. 2024 Oct;17(5):1128-1140. doi: 10.1016/j.mucimm.2024.06.005. Epub 2024 Jun 19. Mucosal Immunol. 2024. PMID: 38906220 Review.
-
Ethyl Acetate Fraction from Eucommia ulmoides Ameliorates Particulate Matter (PM)2.5-Induced Intestinal Damage by Restoring Barrier Integrity and Regulating Inflammatory Responses.J Microbiol Biotechnol. 2025 Jul 18;35:e2504002. doi: 10.4014/jmb.2504.04002. J Microbiol Biotechnol. 2025. PMID: 40730482 Free PMC article.
-
Gut Microbiota Differences in Infants with Cow-Milk-Induced Allergic Proctocolitis: A Comparative Cross-Sectional Study.Children (Basel). 2025 Jun 5;12(6):734. doi: 10.3390/children12060734. Children (Basel). 2025. PMID: 40564692 Free PMC article.
-
Scientific developments in understanding food allergy prevention, diagnosis, and treatment.Front Immunol. 2025 Apr 22;16:1572283. doi: 10.3389/fimmu.2025.1572283. eCollection 2025. Front Immunol. 2025. PMID: 40330465 Free PMC article. Review.
References
-
- Wang Ms J, Kang Ms X, Huang Ms ZQ, Shen Ms L, Luo Md Q, Li Ms MY, et al. . Protease-Activated Receptor-2 Decreased Zonula Occlidens-1 and Claudin-1 Expression and Induced Epithelial Barrier Dysfunction in Allergic Rhinitis. Am J Rhinol Allergy (2021) 35(1):26–35. doi: 10.1177/1945892420932486 - DOI - PubMed

