Seroconversion Rate After SARS-CoV-2 Infection and Two Doses of Either ChAdOx1-nCOV COVISHIELD™ or BBV-152 COVAXIN™ Vaccination in Renal Allograft Recipients: An Experience of Two Public and Private Tertiary Care Center
- PMID: 35844596
- PMCID: PMC9280041
- DOI: 10.3389/fimmu.2022.911738
Seroconversion Rate After SARS-CoV-2 Infection and Two Doses of Either ChAdOx1-nCOV COVISHIELD™ or BBV-152 COVAXIN™ Vaccination in Renal Allograft Recipients: An Experience of Two Public and Private Tertiary Care Center
Abstract
Introduction: Vaccination is an effective strategy for preventing SARS-CoV-2 infection and associated mortality. Renal Transplant Recipients (RTRs) are vulnerable to acquiring infection and high mortality due to their immunocompromised state. Varying responses to the different vaccines, depending on types of vaccines and population, have been reported. Vaccines supply is also limited. The current study evaluated the seroconversion rate after SARS-CoV-2 infection and 2 doses of either COVAXIN™ or COVISHIELD™ vaccination in RTR.
Methods: The serum anti-SARS-CoV-2 spike protein neutralizing antibody titer was measured in 370 RTRs who acquired SARS-CoV-2 infection (n=172), yet not vaccinated; and those vaccinated with COVAXIN™ (n=78), and COVISHIELD™ (n=120) by chemiluminescence microparticle immunoassay methods from serum.
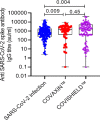
Result: Overall, the seroconversion rate either after vaccination or infection was 85.13% (315/370). The vaccine-associated seroconversion was 80.30% (159/198). SARS-CoV-2 infection-associated seroconversion was 90.69% (156/172), COVISHIELD™ associated seroconversion was 79.2% (95/120), and COVAXIN™ associated seroconversion was 82.05% (64/78). The median IgG titer in the SARS-CoV-2 infection group was 646.50 AU/ml (IQR: 232.52-1717.42), in the COVAXIN™ group was 1449.75 AU/ml (IQR: 400.0-3068.55), and the COVISHIELD™ vaccination group was 1500.51 AU/ml (IQR: 379.47-4938.50). The seroconversion rate and antibody titers were similar irrespective of the place of sampling. Patient's age-associated seroconversion in <45 years was 88.01% (213/242), 45.1-60 years was 83.18% (94/113), and > 60 years was 58.3% (7/12).
Conclusions: Both infection and vaccination induce robust antibody formation in RTRs. The seroconversion rate after SARS-CoV-2 infection was higher but with a lower antibody titer than vaccines. The vaccines, COVAXIN™ and COVISHIELD™, induce more elevated antibody titers than natural infection. The seroconversion rate and antibody titer in Indian RTRs appears to be better than in the western population, irrespective of their vaccination status.
Keywords: COVAXIN™; COVISHIELD™; anti- SARS-CoV-2 antibody; humoral immunity; vaccination.
Copyright © 2022 Prasad, Bansal, Yadav, Manhas, Yadav, Gautam, Kushwaha, Singh, Bhadauria, Yachha, Behera and Kaul.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Evaluation of SARS-CoV-2 Humoral Response Following Vaccination with ChAdOx1 nCoV-19 (Covishield™) and/or Sinopharm, BBIBP-CorV (Vero cell™).J Nepal Health Res Counc. 2024 Mar 22;21(3):523-529. doi: 10.33314/jnhrc.v21i3.4634. J Nepal Health Res Counc. 2024. PMID: 38615227
-
Higher pro-inflammatory cytokines IL-6 and IFN-γ are associated with anti-SARS-CoV-2 spike protein-specific seroconversion in renal allograft recipients.Transpl Infect Dis. 2023 Oct;25(5):e14133. doi: 10.1111/tid.14133. Epub 2023 Aug 21. Transpl Infect Dis. 2023. PMID: 37605477
-
Immunogenicity of SARS-CoV-2 vaccines BBV152 (COVAXIN®) and ChAdOx1 nCoV-19 (COVISHIELD™) in seronegative and seropositive individuals in India: a multicentre, nonrandomised observational study.Lancet Reg Health Southeast Asia. 2024 Feb 27;22:100361. doi: 10.1016/j.lansea.2024.100361. eCollection 2024 Mar. Lancet Reg Health Southeast Asia. 2024. PMID: 38482152 Free PMC article.
-
Immunogenicity of SARS-CoV-2 mRNA vaccine in dialysis and kidney transplant patients: A systematic review.Tuberk Toraks. 2021 Dec;69(4):547-560. doi: 10.5578/tt.20219612. Tuberk Toraks. 2021. PMID: 34957748 English.
-
What has vaccination against COVID-19 in CKD patients taught us?J Nephrol. 2023 Jun;36(5):1257-1266. doi: 10.1007/s40620-023-01640-w. Epub 2023 May 4. J Nephrol. 2023. PMID: 37140817 Free PMC article. Review.
Cited by
-
A longitudinal study on SARS-CoV-2 seroconversion, reinfection and neutralisation spanning several variant waves and vaccination campaigns, Heinsberg, Germany, April 2020 to November 2022.Euro Surveill. 2024 Jun;29(26):2300659. doi: 10.2807/1560-7917.ES.2024.29.26.2300659. Euro Surveill. 2024. PMID: 38940003 Free PMC article.
-
Humoral immune response to an mRNA-1273 booster after chAdOx1-nCoV-19-priming among patients undergoing hemodialysis.Tzu Chi Med J. 2023 Oct 6;35(4):343-347. doi: 10.4103/tcmj.tcmj_107_23. eCollection 2023 Oct-Dec. Tzu Chi Med J. 2023. PMID: 38035061 Free PMC article.
-
Estimation of SARS-CoV-2 IgG Antibodies in Healthcare Worker-Administered Covishield and Covaxin Vaccines at a Tertiary Care Hospital in Jharkhand, India.Cureus. 2023 Oct 24;15(10):e47566. doi: 10.7759/cureus.47566. eCollection 2023 Oct. Cureus. 2023. PMID: 38021860 Free PMC article.
-
Association of Human Leucocyte Antigen Polymorphism with Coronavirus Disease 19 in Renal Transplant Recipients.Vaccines (Basel). 2022 Oct 30;10(11):1840. doi: 10.3390/vaccines10111840. Vaccines (Basel). 2022. PMID: 36366349 Free PMC article.
-
Antibody Response to Covishield and Covaxin in Kidney Transplant Recipients.Indian J Nephrol. 2025 Mar-Apr;35(2):277-282. doi: 10.25259/ijn_549_23. Epub 2024 Aug 1. Indian J Nephrol. 2025. PMID: 40060063 Free PMC article.
References
-
- Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, et al. . Antibody Response After First and Second-Dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) Among Health Care Workers in India: The Final Results of Cross-Sectional Coronavirus Vaccine-Induced Antibody Titre (COVAT) Study. Vaccine (2021) 39:6492–509. doi: 10.1016/j.vaccine.2021.09.055 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous