TREGking From Gut to Brain: The Control of Regulatory T Cells Along the Gut-Brain Axis
- PMID: 35844606
- PMCID: PMC9279871
- DOI: 10.3389/fimmu.2022.916066
TREGking From Gut to Brain: The Control of Regulatory T Cells Along the Gut-Brain Axis
Abstract
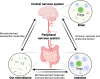
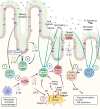
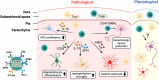
The human gastrointestinal tract has an enormous and diverse microbial community, termed microbiota, that is necessary for the development of the immune system and tissue homeostasis. In contrast, microbial dysbiosis is associated with various inflammatory and autoimmune diseases as well as neurological disorders in humans by affecting not only the immune system in the gastrointestinal tract but also other distal organs. FOXP3+ regulatory T cells (Tregs) are a subset of CD4+ helper T cell lineages that function as a gatekeeper for immune activation and are essential for peripheral autoimmunity prevention. Tregs are crucial to the maintenance of immunological homeostasis and tolerance at barrier regions. Tregs reside in both lymphoid and non-lymphoid tissues, and tissue-resident Tregs have unique tissue-specific phenotype and distinct function. The gut microbiota has an impact on Tregs development, accumulation, and function in periphery. Tregs, in turn, modulate antigen-specific responses aimed towards gut microbes, which supports the host-microbiota symbiotic interaction in the gut. Recent studies have indicated that Tregs interact with a variety of resident cells in central nervous system (CNS) to limit the progression of neurological illnesses such as ischemic stroke, Alzheimer's disease, and Parkinson's disease. The gastrointestinal tract and CNS are functionally connected, and current findings provide insights that Tregs function along the gut-brain axis by interacting with immune, epithelial, and neuronal cells. The purpose of this study is to explain our current knowledge of the biological role of tissue-resident Tregs, as well as the interaction along the gut-brain axis.
Keywords: central nervous system; gastrointestinal tract; gut–brain axis; microbiota; neuroimmune; regulatory T cell.
Copyright © 2022 Choi, Kim, Akuzum, Chang, Lee and Kwon.
Conflict of interest statement
H-KK is a scientific advisor member in Enhanced Neo Cell. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology.Front Immunol. 2020 Dec 10;11:604179. doi: 10.3389/fimmu.2020.604179. eCollection 2020. Front Immunol. 2020. PMID: 33362788 Free PMC article. Review.
-
The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders.Cells. 2022 Dec 23;12(1):54. doi: 10.3390/cells12010054. Cells. 2022. PMID: 36611848 Free PMC article. Review.
-
Gut microbiota in regulatory T cell generation and function: mechanisms and health implications.Gut Microbes. 2025 Dec;17(1):2516702. doi: 10.1080/19490976.2025.2516702. Epub 2025 Jun 15. Gut Microbes. 2025. PMID: 40517372 Free PMC article. Review.
-
Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle.Pharmacol Ther. 2022 Mar;231:107988. doi: 10.1016/j.pharmthera.2021.107988. Epub 2021 Sep 16. Pharmacol Ther. 2022. PMID: 34536490 Review.
-
The Gut-Brain Axis in Multiple Sclerosis. Is Its Dysfunction a Pathological Trigger or a Consequence of the Disease?Front Immunol. 2021 Sep 21;12:718220. doi: 10.3389/fimmu.2021.718220. eCollection 2021. Front Immunol. 2021. PMID: 34621267 Free PMC article. Review.
Cited by
-
The interaction between rumen microbiota and neurotransmitters plays an important role in the adaptation of phenological changes in Tibetan sheep.BMC Vet Res. 2025 May 26;21(1):373. doi: 10.1186/s12917-025-04823-8. BMC Vet Res. 2025. PMID: 40414864 Free PMC article.
-
The Interplay between Gut Microbiota and Parkinson's Disease: Implications on Diagnosis and Treatment.Int J Mol Sci. 2022 Oct 14;23(20):12289. doi: 10.3390/ijms232012289. Int J Mol Sci. 2022. PMID: 36293176 Free PMC article. Review.
-
Short-chain fatty acids are a key mediator of gut microbial regulation of T cell trafficking and differentiation after traumatic brain injury.Res Sq [Preprint]. 2024 Nov 21:rs.3.rs-5397327. doi: 10.21203/rs.3.rs-5397327/v1. Res Sq. 2024. Update in: Exp Neurol. 2025 Oct;392:115349. doi: 10.1016/j.expneurol.2025.115349. PMID: 39606443 Free PMC article. Updated. Preprint.
-
Bibliometric analysis of the gut microbiota and stroke from 2002 to 2022.Heliyon. 2024 May 3;10(9):e30424. doi: 10.1016/j.heliyon.2024.e30424. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765104 Free PMC article.
-
Amyloid, Crohn's disease, and Alzheimer's disease - are they linked?Front Cell Infect Microbiol. 2024 May 8;14:1393809. doi: 10.3389/fcimb.2024.1393809. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38779559 Free PMC article. Review.
References
-
- Westfall S, Caracci F, Zhao DY, Wu QL, Frolinger T, Simon J, et al. . Microbiota Metabolites Modulate the T Helper 17 to Regulatory T Cell (Th17/Treg) Imbalance Promoting Resilience to Stress-Induced Anxiety- and Depressive-Like Behaviors. Brain Behav Immun (2021) 91:350–68. doi: 10.1016/j.bbi.2020.10.013 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

