Human Milk Oligosaccharides Impact Cellular and Inflammatory Gene Expression and Immune Response
- PMID: 35844612
- PMCID: PMC9278088
- DOI: 10.3389/fimmu.2022.907529
Human Milk Oligosaccharides Impact Cellular and Inflammatory Gene Expression and Immune Response
Abstract
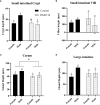
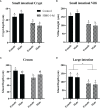
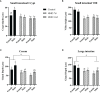
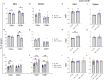
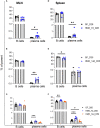
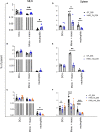
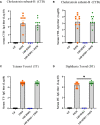
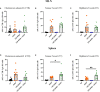
Human milk harbors complex carbohydrates, including human milk oligosaccharides (HMOs), the third most abundant component after lactose and lipids. HMOs have been shown to impact intestinal microbiota, modulate the intestinal immune response, and prevent pathogenic bacterial binding by serving as decoy receptors. However, the direct effect of HMOs on intestinal function and immunity remains to be elucidated. To address this knowledge gap, 21-day-old germ-free mice (C57BI/6) were orally gavaged with 15 mg/day of pooled HMOs for 7 or 14 days and euthanized at day 28 or 35. A set of mice was maintained until day 50 to determine the persistent effects of HMOs. Control groups were maintained in the isolators for 28, 35, or 50 days of age. At the respective endpoints, intestinal tissues were subjected to histomorphometric and transcriptomic analyses, while the spleen and mesenteric lymph nodes (MLNs) were subjected to flow cytometric analysis. The small intestine (SI) crypt was reduced after HMO treatment relative to control at days 28 and 35, while the SI villus height and large intestine (LI) gland depth were decreased in the HMO-treated mice relative to the control at day 35. We report significant HMO-induced and location-specific gene expression changes in host intestinal tissues. HMO treatment significantly upregulated genes involved in extracellular matrix, protein ubiquitination, nuclear transport, and mononuclear cell differentiation. CD4+ T cells were increased in both MLNs and the spleen, while CD8+ T cells were increased in the spleen at day 50 in the HMO group in comparison to controls. In MLNs, plasma cells were increased in HMO group at days 28 and 35, while in the spleen, only at day 28 relative to controls. Macrophages/monocytes and neutrophils were lower in the spleen of the HMO group at days 28, 35, and 50, while in MLNs, only neutrophils were lower at day 50 in the 14-day HMO group. In addition, diphtheria toxoid and tetanus toxoid antibody-secreting cells were higher in HMO-supplemented group compared to controls. Our data suggest that HMOs have a direct effect on gastrointestinal tract metabolism and the immune system even in the absence of host microbiota.
Keywords: HMO; gastrointestinal tract; human milk oligosaccharides; immunity; neonatal.
Copyright © 2022 Rosa, Sharma, Gurung, Casero, Matazel, Bode, Simecka, Elolimy, Tripp, Randolph, Hand, Williams, LeRoith and Yeruva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Microbiota from human infants consuming secretors or non-secretors mothers' milk impacts the gut and immune system in mice.mSystems. 2024 Apr 16;9(4):e0029424. doi: 10.1128/msystems.00294-24. Epub 2024 Mar 26. mSystems. 2024. PMID: 38530054 Free PMC article.
-
Dietary Human Milk Oligosaccharides but Not Prebiotic Oligosaccharides Increase Circulating Natural Killer Cell and Mesenteric Lymph Node Memory T Cell Populations in Noninfected and Rotavirus-Infected Neonatal Piglets.J Nutr. 2017 Jun;147(6):1041-1047. doi: 10.3945/jn.116.243774. Epub 2017 May 10. J Nutr. 2017. PMID: 28490677 Free PMC article.
-
Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs.J Nutr Biochem. 2017 Feb;40:141-154. doi: 10.1016/j.jnutbio.2016.10.011. Epub 2016 Nov 4. J Nutr Biochem. 2017. PMID: 27889684
-
Beneficial effects of human milk oligosaccharides on gut microbiota.Benef Microbes. 2014 Sep;5(3):273-83. doi: 10.3920/BM2013.0080. Benef Microbes. 2014. PMID: 24913838 Review.
-
Gold standard for nutrition: a review of human milk oligosaccharide and its effects on infant gut microbiota.Microb Cell Fact. 2021 May 28;20(1):108. doi: 10.1186/s12934-021-01599-y. Microb Cell Fact. 2021. PMID: 34049536 Free PMC article. Review.
Cited by
-
Interactions of human milk oligosaccharides with the immune system.Front Immunol. 2025 Jan 14;15:1523829. doi: 10.3389/fimmu.2024.1523829. eCollection 2024. Front Immunol. 2025. PMID: 39877362 Free PMC article. Review.
-
The Impact of Human Milk Oligosaccharides on Antibiotic-Induced Microbial Dysbiosis and Gut Inflammation in Mice.Antibiotics (Basel). 2025 May 10;14(5):488. doi: 10.3390/antibiotics14050488. Antibiotics (Basel). 2025. PMID: 40426555 Free PMC article.
-
Exploring galectin interactions with human milk oligosaccharides and blood group antigens identifies BGA6 as a functional galectin-4 ligand.J Biol Chem. 2024 Aug;300(8):107573. doi: 10.1016/j.jbc.2024.107573. Epub 2024 Jul 14. J Biol Chem. 2024. PMID: 39009340 Free PMC article.
-
Formula supplementation with human and bovine milk oligosaccharides modulates blood IgG and T-helper cell populations, and ex vivo LPS-stimulated cytokine production in a neonatal preclinical model.Front Immunol. 2023 Dec 20;14:1327853. doi: 10.3389/fimmu.2023.1327853. eCollection 2023. Front Immunol. 2023. PMID: 38179055 Free PMC article.
-
Specific Human Milk Oligosaccharides Differentially Promote Th1 and Regulatory Responses in a CpG-Activated Epithelial/Immune Cell Coculture.Biomolecules. 2023 Jan 31;13(2):263. doi: 10.3390/biom13020263. Biomolecules. 2023. PMID: 36830632 Free PMC article.
References
-
- Sobti J, Mathur GP, Gupta A. WHO's Proposed Global Strategy for Infant and Young Child Feeding: A Viewpoint. J Indian Med Assoc (2002) 100(8):502–4, 6. - PubMed
-
- Eidelman AI., Schanler RJ., Johnston M, Landers S, Noble L, Szucs K. Breastfeeding and the Use of Human Milk. Pediatrics (2012) 129(3):e827–41. doi: doi.org/10.1542/peds.2011-3552 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

