Non-Palladium-Catalyzed Oxidative Coupling Reactions Using Hypervalent Iodine Reagents
- PMID: 35844643
- PMCID: PMC9283985
- DOI: 10.3389/fchem.2022.909250
Non-Palladium-Catalyzed Oxidative Coupling Reactions Using Hypervalent Iodine Reagents
Abstract
Transition metal-catalyzed direct oxidative coupling reactions via C-H bond activation have emerged as a straightforward strategy for the construction of complex molecules in organic synthesis. The direct transformation of C-H bonds into carbon-carbon and carbon-heteroatom bonds renders the requirement of prefunctionalization of starting materials and, therefore, represents a more efficient alternative to the traditional cross-coupling reactions. The key to the unprecedented progress made in this area has been the identification of an appropriate oxidant that facilitates oxidation and provides heteroatom ligands at the metal center. In this context, hypervalent iodine compounds have evolved as mainstream reagents particularly because of their excellent oxidizing nature, high electrophilicity, and versatile reactivity. They are environmentally benign reagents, stable, non-toxic, and relatively cheaper than inorganic oxidants. For many years, palladium catalysis has dominated these oxidative coupling reactions, but eventually, other transition metal catalysts such as gold, copper, platinum, iron, etc. were found to be promising alternate catalysts for facilitating such reactions. This review article critically summarizes the recent developments in non-palladium-catalyzed oxidative coupling reactions mediated by hypervalent iodine (III) reagents with significant emphasis on understanding the mechanistic aspects in detail.
Keywords: catalyst; copper; gold; hypervalent iodine reagents; oxidant; oxidative coupling.
Copyright © 2022 Shetgaonkar, Raju, China, Takenaga, Dohi and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

















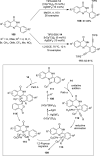
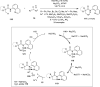
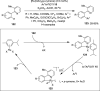
References
Publication types
LinkOut - more resources
Full Text Sources

