Oriented External Electric Fields Regurating the Reaction Mechanism of CH4 Oxidation Catalyzed by Fe(IV)-Oxo-Corrolazine: Insight from Density Functional Calculations
- PMID: 35844657
- PMCID: PMC9277104
- DOI: 10.3389/fchem.2022.896944
Oriented External Electric Fields Regurating the Reaction Mechanism of CH4 Oxidation Catalyzed by Fe(IV)-Oxo-Corrolazine: Insight from Density Functional Calculations
Abstract
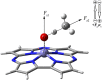
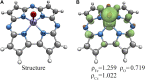
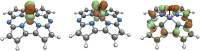
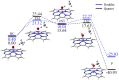
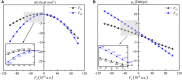
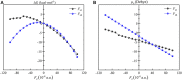
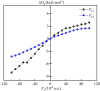
Methane is the simplest alkane and can be used as an alternative energy source for oil and coal, but the greenhouse effect caused by its leakage into the air is not negligible, and its conversion into liquid methanol not only facilitates transportation, but also contributes to carbon neutrality. In order to find an efficient method for converting methane to methanol, CH4 oxidation catalyzed by Fe(IV)-Oxo-corrolazine (Fe(IV)-Oxo-Cz) and its reaction mechanism regulation by oriented external electric fields (OEEFs) are systematically studied by density functional calculations. The calculations show that Fe(IV)-Oxo-Cz can abstract one H atom from CH4 to form the intermediate with OH group connecting on the corrolazine ring, with the energy barrier of 25.44 kcal mol-1. And then the product methanol is formed through the following rebound reaction. Moreover, the energy barrier can be reduced to 20.72 kcal mol-1 through a two-state reaction pathway. Furthermore, the effect of OEEFs on the reaction is investigated. We found that OEEFs can effectively regulate the reaction by adjusting the stability of the reactant and the transition state through the interaction of electric field-molecular dipole moment. When the electric field is negative, the energy barrier of the reaction decreases with the increase of electric intensity. Moreover, the OEEF aligned along the intrinsic Fe‒O reaction axis can effectively regulate the ability of forming the OH on the corrolazine ring by adjusting the charges of O and H atoms. When the electric field intensity is -0.010 a.u., the OH can be directly rebounded to the CH3· before it is connecting on the corrolazine ring, thus forming the product directly from the transition state without passing through the intermediate with only an energy barrier of 17.34 kcal mol-1, which greatly improves the selectivity of the reaction.
Keywords: CH4 oxidation; Fe(IV)-Oxo-Corrolazine; catalysis; density functional calculations; oriented external electric fields.
Copyright © 2022 Wu, Long, Wang, Liang and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Baglia R. A., Prokop-Prigge K. A., Neu H. M., Siegler M. A., Goldberg D. P. (2015). Mn(V)(O) versus Cr(V)(O) Porphyrinoid Complexes: Structural Characterization and Implications for Basicity Controlling H-Atom Abstraction. J. Am. Chem. Soc. 137, 10874–10877. 10.1021/jacs.5b05142 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

