Clinical Application Value of Pharmacokinetic Parameters of Vancomycin in Children Treated in the Pediatric Intensive Care Unit
- PMID: 35844752
- PMCID: PMC9279905
- DOI: 10.3389/fped.2022.867712
Clinical Application Value of Pharmacokinetic Parameters of Vancomycin in Children Treated in the Pediatric Intensive Care Unit
Abstract
Objective: To explore the efficacy and safety of vancomycin as measured by pharmacokinetic/pharmacodynamic parameters in children with severe infection in the Pediatric Intensive Care Unit (PICU) and to determine the appropriate threshold for avoiding nephrotoxicity.
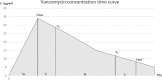
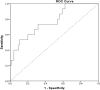
Methods: The medical records of hospitalized children with severe infection treated with vancomycin in the PICU of a tertiary pediatric hospital from September 2018 to January 2021 were retrospectively collected. Univariate analysis was used to assess the correlation between vancomycin pharmacokinetic/pharmacodynamic parameters and therapeutic efficacy or vancomycin-related nephrotoxicity. Binary logistic regression was used to analyze the risk factors for vancomycin-related nephrotoxicity. The vancomycin area under the concentration-time curve over 24 h (AUC0-24) threshold was determined by receiver operating characteristic (ROC) curve analysis.
Results: One hundred and 10 patients were included in this study. Seventy-six patients (69.1%) exhibited clinically effective response, while the rest exhibited clinically ineffective response. There were no significant differences in APACHE II score, steady-state trough concentration, peak concentration or AUC0-24 of vancomycin between the effective and ineffective groups. Among the 110 patients, vancomycin-related nephrotoxicity occurred in 15 patients (13.6%). Multivariate analysis showed that vancomycin treatment duration, trough concentration, and AUC0-24 were risk factors for vancomycin-related nephrotoxicity. The ROC curve indicated that AUC0-24 < 537.18 mg.h/L was a suitable cutoff point for predicting vancomycin-related nephrotoxicity.
Conclusion: No significant correlations were found between the trough concentration or AUC0-24 of vancomycin and therapeutic efficacy when the daily dose of vancomycin was approximately 40 mg/kg d, while the trough concentration and AUC0-24 were both closely related to vancomycin-related nephrotoxicity. The combination of AUC0-24 and trough concentration for therapeutic drug monitoring may reduce the risk of nephrotoxicity.
Keywords: children; nephrotoxicity; pharmacokinetics/pharmacodynamics; therapeutic drug monitoring; vancomycin.
Copyright © 2022 Zhou, Xiong, Bai, Dang, Li, Xu, Fu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Arroyo-Mercado F, Khudyakov A, Chawla GS, Cantres-Fonseca O, McFarlane IM. Red man syndrome with oral vancomycin: a case report. Am J Med Case Rep. (2019) 7:16–7. 10.12691/ajmcr-7-1-5 - DOI
-
- Martel TJ, Jamil RT, King KC. Vancomycin Flushing Syndrome. Treasure Island, FL: StatPearls Publishing; (2021). - PubMed
LinkOut - more resources
Full Text Sources

