Safety and efficacy of mesenchymal stem cells in severe/critical patients with COVID-19: A systematic review and meta-analysis
- PMID: 35844767
- PMCID: PMC9270852
- DOI: 10.1016/j.eclinm.2022.101545
Safety and efficacy of mesenchymal stem cells in severe/critical patients with COVID-19: A systematic review and meta-analysis
Abstract
Background: The present study aims to better understand the efficacy and safety of mesenchymal stromal cells (MSCs) in treating severe/critical patients with COVID-19.
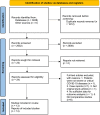
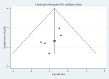
Methods: PubMed, the Cochrane Library, and the Chinese electronic database CNKI were searched from inception up to Dec 19, 2021. Original comparative studies for MSC treatment + standard treatment for severe/critical patients with COVID-19, with placebo or standard treatment as the control group, were included. The primary outcomes were in-hospital mortality and adverse events (AEs). A meta-analysis was performed to compare the mortality rates between the two groups. Then, a subgroup analysis was performed according to the category of the disease (severe or critical) and MSC dose. Afterwards, a descriptive analysis was performed for AEs and secondary outcomes. The funnel plot and Egger's test were used for the publication bias assessment.
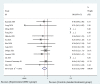
Findings: Compared to placebo or standard care, MSCs provide significant benefit in the treatment of patients with severe/critical COVID-19, in terms of in-hospital mortality rate (odds ratio: 0.52, 95% CI 0.32-0.84), with very low heterogeneity (P=0.998 [Q test], I 2=0.0%) and less AEs. No significant difference was found in mortality rate due to the different disease categories or MSC doses. Furthermore, no publication bias was found.
Interpretation: The present study demonstrates that MSCs are highly likely to reduce mortality and are safe to use for patients with severe or critical COVID-19, regardless of whether 1-3 doses are applied. However, due to the small sample size of the included studies, further high-quality, large-scale trials are needed to confirm this statement in the future.
Funding: The National Key Research and Development Program of China (No. 2020YFC0860900), the Science and Technology Project of Wuhan (No. 2020020602012112), the Tianjin Science and Technology Research Program (18PTSYJC00070 and 16PTWYHZ00030), Haihe Laboratory of Cell Ecosystem Innovation Fund (HH22KYZX0046), and the Tianjin Free Trade Zone Innovation Development Project (ZMCY-03-2021002-01) funded the study. We are also grateful for the support from the 3551 Talent Plan of China Optics Valley.
Keywords: Cellular therapy; Coronavirus disease-19 (COVID-19); Mesenchymal stem cells; Meta-analysis; Systematic review.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Human mesenchymal stem cell therapy in severe COVID-19 patients: 2-year follow-up results of a randomized, double-blind, placebo-controlled trial.EBioMedicine. 2023 Jun;92:104600. doi: 10.1016/j.ebiom.2023.104600. Epub 2023 May 5. EBioMedicine. 2023. PMID: 37149930 Free PMC article. Clinical Trial.
-
Research Status of the Safety and Efficacy of Mesenchymal Stem Cells in the Treatment of COVID-19-Related Pneumonia: A Systematic Review and Meta-Analysis.Stem Cells Dev. 2021 Oct 1;30(19):947-969. doi: 10.1089/scd.2021.0179. Stem Cells Dev. 2021. PMID: 34416823
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Efficacy and safety of mesenchymal stem cells for the treatment of patients infected with COVID-19: a systematic review and meta-analysis protocol.BMJ Open. 2020 Dec 18;10(12):e042085. doi: 10.1136/bmjopen-2020-042085. BMJ Open. 2020. PMID: 33371042 Free PMC article.
-
The clinical efficacy and safety of mesenchymal stromal cells for patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials.J Infect Public Health. 2022 Aug;15(8):896-901. doi: 10.1016/j.jiph.2022.07.001. Epub 2022 Jul 7. J Infect Public Health. 2022. PMID: 35849852 Free PMC article.
Cited by
-
Mesenchymal Stem Cell Priming: Potential Benefits of Administration of Molecular Hydrogen.Pharmaceuticals (Basel). 2024 Apr 7;17(4):469. doi: 10.3390/ph17040469. Pharmaceuticals (Basel). 2024. PMID: 38675429 Free PMC article. Review.
-
Cancer and COVID-19: unravelling the immunological interplay with a review of promising therapies against severe SARS-CoV-2 for cancer patients.J Hematol Oncol. 2023 Apr 13;16(1):39. doi: 10.1186/s13045-023-01432-6. J Hematol Oncol. 2023. PMID: 37055774 Free PMC article. Review.
-
Efficacy and safety of mesenchymal stem cells in knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials.Stem Cell Res Ther. 2025 Mar 7;16(1):122. doi: 10.1186/s13287-025-04252-2. Stem Cell Res Ther. 2025. PMID: 40055739 Free PMC article.
-
Efficacy of Wharton Jelly Mesenchymal Stromal Cells infusions in moderate to severe SARS-Cov-2 related acute respiratory distress syndrome: a phase 2a double-blind randomized controlled trial.Front Med (Lausanne). 2023 Aug 29;10:1224865. doi: 10.3389/fmed.2023.1224865. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37706025 Free PMC article.
-
Stem cell therapy for COVID-19 treatment: an umbrella review.Int J Surg. 2024 Oct 1;110(10):6402-6417. doi: 10.1097/JS9.0000000000001786. Int J Surg. 2024. PMID: 38967503 Free PMC article.
References
-
- World Health Organization (WHO) Coronavirus (COVID-19) Dashboard. 9 December 2021. https://covid19.who.int/. Accessed 10 December 2021.
-
- Ghelichi-Ghojogh M, Allah Kalteh E, Fararooei M. Coronavirus disease 2019; epidemiology and recommendations. J Prev Epidemiol. 2020;5:e01.
-
- Berlin DA, Gulick RM, Martinez FJ. Severe covid-19. N Engl J Med. 2020;383:2451–2460. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

