Enhanced seedling growth by 3- n-pentadecylphenolethanolamide is mediated by fatty acid amide hydrolases in upland cotton (Gossypium hirsutum L.)
- PMID: 35844778
- PMCID: PMC9277032
- DOI: 10.1002/pld3.421
Enhanced seedling growth by 3- n-pentadecylphenolethanolamide is mediated by fatty acid amide hydrolases in upland cotton (Gossypium hirsutum L.)
Abstract
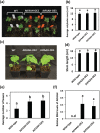
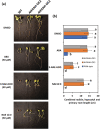
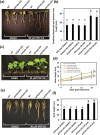
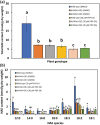
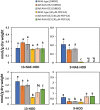
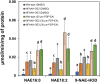
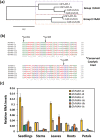
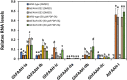
Fatty acid amide hydrolase (FAAH) is a conserved amidase that is known to modulate the levels of endogenous N-acylethanolamines (NAEs) in both plants and animals. The activity of FAAH is enhanced in vitro by synthetic phenoxyacylethanolamides resulting in greater hydrolysis of NAEs. Previously, 3-n-pentadecylphenolethanolamide (PDP-EA) was shown to exert positive effects on the development of Arabidopsis seedlings by enhancing Arabidopsis FAAH (AtFAAH) activity. However, there is little information regarding FAAH activity and the impact of PDP-EA in the development of seedlings of other plant species. Here, we examined the effects of PDP-EA on growth of upland cotton (Gossypium hirsutum L. cv Coker 312) seedlings including two lines of transgenic seedlings overexpressing AtFAAH. Independent transgenic events showed accelerated true-leaf emergence compared with non-transgenic controls. Exogenous applications of PDP-EA led to increases in overall seedling growth in AtFAAH transgenic lines. These enhanced-growth phenotypes coincided with elevated FAAH activities toward NAEs and NAE oxylipins. Conversely, the endogenous contents of NAEs and NAE-oxylipin species, especially linoleoylethanolamide and 9-hydroxy linoleoylethanolamide, were lower in PDP-EA treated seedlings than in controls. Further, transcripts for endogenous cotton FAAH genes were increased following PDP-EA exposure. Collectively, our data corroborate that the enhancement of FAAH enzyme activity by PDP-EA stimulates NAE-hydrolysis and that this results in enhanced growth in seedlings of a perennial crop species, extending the role of NAE metabolism in seedling development beyond the model annual plant species, Arabidopsis thaliana.
Keywords: 3‐n‐pentadecylphenolethanolamide; N‐acylethanolamines; cotton; fatty acid amide hydrolase; oxylipins; seedling growth.
© 2022 The Authors. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Fatty acid amide hydrolase and 9-lipoxygenase modulate cotton seedling growth by ethanolamide oxylipin levels.Plant Physiol. 2023 Feb 12;191(2):1234-1253. doi: 10.1093/plphys/kiac556. Plant Physiol. 2023. PMID: 36472510 Free PMC article.
-
Manipulation of Arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines.Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):12197-202. doi: 10.1073/pnas.0603571103. Epub 2006 Jul 31. Proc Natl Acad Sci U S A. 2006. PMID: 16880402 Free PMC article.
-
Lipoxygenase-mediated oxidation of polyunsaturated N-acylethanolamines in Arabidopsis.J Biol Chem. 2011 Apr 29;286(17):15205-14. doi: 10.1074/jbc.M110.217588. Epub 2011 Mar 3. J Biol Chem. 2011. PMID: 21372125 Free PMC article.
-
The N-acylethanolamine-mediated regulatory pathway in plants.Chem Biodivers. 2007 Aug;4(8):1933-55. doi: 10.1002/cbdv.200790161. Chem Biodivers. 2007. PMID: 17712835 Review.
-
Fatty Acid Amide Hydrolases: An Expanded Capacity for Chemical Communication?Trends Plant Sci. 2020 Mar;25(3):236-249. doi: 10.1016/j.tplants.2019.11.002. Epub 2020 Jan 7. Trends Plant Sci. 2020. PMID: 31919033 Review.
Cited by
-
Discovering novel genomic regions explaining adaptation of bread wheat to conservation agriculture through GWAS.Sci Rep. 2024 Jul 16;14(1):16351. doi: 10.1038/s41598-024-66903-3. Sci Rep. 2024. PMID: 39013994 Free PMC article.
-
Fatty acid amide hydrolase and 9-lipoxygenase modulate cotton seedling growth by ethanolamide oxylipin levels.Plant Physiol. 2023 Feb 12;191(2):1234-1253. doi: 10.1093/plphys/kiac556. Plant Physiol. 2023. PMID: 36472510 Free PMC article.
-
Two legume fatty acid amide hydrolase isoforms with distinct preferences for microbial- and plant-derived acylamides.Sci Rep. 2023 May 9;13(1):7486. doi: 10.1038/s41598-023-34754-z. Sci Rep. 2023. PMID: 37161076 Free PMC article.
References
-
- Amack, S. C. , & Antunes, M. S. (2020). CaMV35S promoter—A plant biology and biotechnology workhorse in the era of synthetic biology. Current Plant Biology, 24, 100179.
-
- Aziz, M. , & Chapman, K. D. (2020). Fatty acid amide hydrolases: An expanded capacity for chemical communication? Trends in Plant Science, 25(3), 236–249. - PubMed
-
- Blancaflor, E. B. , Kilaru, A. , Keereetaweep, J. , Khan, B. R. , Faure, L. , & Chapman, K. D. (2014). N‐Acylethanolamines: Lipid metabolites with functions in plant growth and development. The Plant Journal, 79(4), 568–583. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

