Spatiotemporal quantification of metastatic tumour cell growth and distribution in lymph nodes by whole-mount tissue 3D imaging
- PMID: 35844788
- PMCID: PMC9274482
- DOI: 10.7150/ijbs.72552
Spatiotemporal quantification of metastatic tumour cell growth and distribution in lymph nodes by whole-mount tissue 3D imaging
Abstract
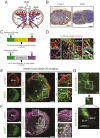
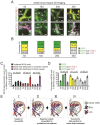
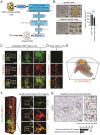
Lymph nodes (LNs) are a common site of metastasis in many solid cancers. Tumour cells can migrate to LNs for further metastatic colonization of distant organs, indicating poor prognosis and requiring different clinical interventions. The histopathological diagnostic methods currently used to detect clinical lymph node metastasis (LNM) have limitations, such as incomplete visualization. To obtain a complete picture of metastatic LNs on the spatial and temporal scales, we used ultimate 3D imaging of solvent-cleared organs (uDISCO) and 3D rapid immunostaining. MC38 cells labelled with EGFP were injected into the left footpads of C57BL/6 mice. Draining lymph nodes (DLNs) harvested from these mice were cleared using the uDISCO method. Metastatic colorectal cancer (CRC) cells in various regions of DLNs from mice at different time points were quantified using 3D imaging of whole-mount tissue. Several stages of tumour cell growth and distribution in LNs were identified: 1) invasion of lymphatic vessels (LVs) and blood vessels (BVs); 2) dispersion outside LVs and BVs for proliferation and expansion; and 3) re-entry into BVs and efferent lymphatic vessels (ELVs) for further distant metastasis. Moreover, these data demonstrated that mouse fibroblast cells (MFCs) could not only promote LNM of tumour cells but also metastasize to LNs together with tumour cells, thus providing a "soil" for tumour cell colonization. In conclusion, 3D imaging of whole-mount tissue and spatiotemporal analysis of LNM may collectively constitute an auxiliary method to improve the accuracy of clinical LNM detection.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Study of fluid dynamics reveals direct communications between lymphatic vessels and venous blood vessels at lymph nodes of mice.J Immunol Methods. 2017 Jun;445:1-9. doi: 10.1016/j.jim.2017.02.008. Epub 2017 Feb 22. J Immunol Methods. 2017. PMID: 28237707
-
Imaging tumor-induced sentinel lymph node lymphangiogenesis with LyP-1 peptide.Amino Acids. 2012 Jun;42(6):2343-51. doi: 10.1007/s00726-011-0976-1. Epub 2011 Jul 19. Amino Acids. 2012. PMID: 21769497 Free PMC article.
-
Mouse model of lymph node metastasis via afferent lymphatic vessels for development of imaging modalities.PLoS One. 2013;8(2):e55797. doi: 10.1371/journal.pone.0055797. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405215 Free PMC article.
-
Tumor Regulation of Lymph Node Lymphatic Sinus Growth and Lymph Flow in Mice and in Humans.Yale J Biol Med. 2017 Sep 25;90(3):403-415. eCollection 2017 Sep. Yale J Biol Med. 2017. PMID: 28955180 Free PMC article. Review.
-
Cellular traffic through afferent lymphatic vessels.Vascul Pharmacol. 2019 Jan;112:31-41. doi: 10.1016/j.vph.2018.08.001. Epub 2018 Aug 7. Vascul Pharmacol. 2019. PMID: 30092362 Review.
Cited by
-
Prognostic and predictive value of tumor infiltration proportion within lymph nodes in N1 colorectal cancer.Front Oncol. 2025 Mar 25;15:1512960. doi: 10.3389/fonc.2025.1512960. eCollection 2025. Front Oncol. 2025. PMID: 40201345 Free PMC article.
-
TNS4 promotes lymph node metastasis of gastric cancer by interacting with integrin Β1 and inducing the activation of fibroblastic reticular cell.Cancer Cell Int. 2025 Jun 6;25(1):204. doi: 10.1186/s12935-025-03830-x. Cancer Cell Int. 2025. PMID: 40481538 Free PMC article.
-
Assessing the clinical utility of tumor invasion proportion of lymph nodes for enhanced risk stratification in N1 colorectal cancer.Am J Cancer Res. 2024 Dec 15;14(12):5826-5838. doi: 10.62347/DFXC4525. eCollection 2024. Am J Cancer Res. 2024. PMID: 39803664 Free PMC article.
References
-
- Brown M, Assen FP, Leithner A. et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408–1411. - PubMed
-
- Lee CK, Jeong SH, Jang C. et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–649. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

