Gut microbiome in gastrointestinal cancer: a friend or foe?
- PMID: 35844804
- PMCID: PMC9274484
- DOI: 10.7150/ijbs.69331
Gut microbiome in gastrointestinal cancer: a friend or foe?
Abstract
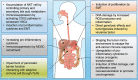
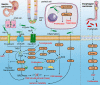
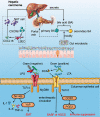
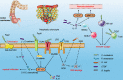
The impact of the gut microbiome on host health is becoming increasingly recognized. To date, there is growing evidence that the complex characteristics of the microbial community play key roles as potential biomarkers and predictors of responses in cancer therapy. Many studies have shown that altered commensal bacteria lead to cancer susceptibility and progression in diverse pathways. In this review, we critically assess the data for gut microbiota related to gastrointestinal cancer, including esophageal, gastric, pancreatic, colorectal cancer, hepatocellular carcinoma and cholangiocarcinoma. Importantly, the underlying mechanisms of gut microbiota involved in cancer occurrence, prevention and treatment are elucidated. The purpose of this review is to provide novel insights for applying this understanding to the development of new therapeutic strategies in gastrointestinal cancer by targeting the microbial community.
Keywords: GI cancer; carcinogenesis; chemotherapy; gut microbiota.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28(Suppl 4):9–17. - PubMed
-
- Allen-Vercoe E, Coburn B. A Microbiota-Derived Metabolite Augments Cancer Immunotherapy Responses in Mice. Cancer Cell. 2020;38:452–3. - PubMed
-
- Ocvirk S, O'Keefe SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 2020. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

