CCDC65, a Gene Knockout that leads to Early Death of Mice, acts as a potentially Novel Tumor Suppressor in Lung Adenocarcinoma
- PMID: 35844805
- PMCID: PMC9274497
- DOI: 10.7150/ijbs.69332
CCDC65, a Gene Knockout that leads to Early Death of Mice, acts as a potentially Novel Tumor Suppressor in Lung Adenocarcinoma
Abstract
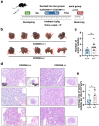
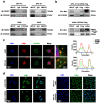
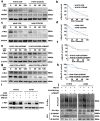
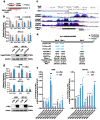
CCDC65 is a member of the coiled-coil domain-containing protein family and was only reported in gastric cancer by our group. We first observed that it is downregulated in lung adenocarcinoma based on the TCGA database. Reduced CCDC65 protein was shown as an unfavorable factor promoting the clinical progression in lung adenocarcinoma. Subsequently, CCDC65-/- mice were found possibly dead of hydrocephalus. Compared with the CCDC65+/+ mice, the downregulation of CCDC65 in CCDC65+/- mice significantly increased the formation ability of lung cancer induced by urethane. In the subsequent investigation, we observed that CCDC65 functions as a tumor suppressor repressing cell proliferation in vitro and in vivo. Molecular mechanism showed that CCDC65 recruited E3 ubiquitin ligase FBXW7 to induce the ubiquitination degradation of c-Myc, an oncogenic transcription factor in tumors, and reduced c-Myc binding to ENO1 promoter, which suppressed the transcription of ENO1. In addition, CCDC65 also recruited FBXW7 to degrade ENO1 protein by ubiquitinated modulation. The downregulated ENO1 further reduced the phosphorylation activation of AKT1, which thus inactivated the cell cycle signal. Our data demonstrated that CCDC65 is a potential tumor suppressor by recruiting FBWX7 to suppress c-Myc/ENO1-induced cell cycle signal in lung adenocarcinoma.
Keywords: CCDC65; Cell cycle; ENO1; Knockdown mice; Ubiquitin degradation; c-Myc.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
CCDC65 as a new potential tumor suppressor induced by metformin inhibits activation of AKT1 via ubiquitination of ENO1 in gastric cancer.Theranostics. 2021 Jul 13;11(16):8112-8128. doi: 10.7150/thno.54961. eCollection 2021. Theranostics. 2021. PMID: 34335983 Free PMC article.
-
ARMCX1 inhibits lung adenocarcinoma progression by recruiting FBXW7 for c-Myc degradation.Biol Direct. 2024 Sep 16;19(1):82. doi: 10.1186/s13062-024-00532-8. Biol Direct. 2024. PMID: 39285446 Free PMC article.
-
Ampelopsin induces apoptosis by regulating multiple c-Myc/S-phase kinase-associated protein 2/F-box and WD repeat-containing protein 7/histone deacetylase 2 pathways in human lung adenocarcinoma cells.Mol Med Rep. 2015 Jan;11(1):105-12. doi: 10.3892/mmr.2014.2733. Epub 2014 Oct 21. Mol Med Rep. 2015. PMID: 25333250 Free PMC article.
-
The FBXW7-NOTCH interactome: A ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues.Cancer Rep (Hoboken). 2021 Aug;4(4):e1369. doi: 10.1002/cnr2.1369. Epub 2021 Apr 6. Cancer Rep (Hoboken). 2021. PMID: 33822486 Free PMC article. Review.
-
Recent insight into the role of FBXW7 as a tumor suppressor.Semin Cancer Biol. 2020 Dec;67(Pt 2):1-15. doi: 10.1016/j.semcancer.2020.02.017. Epub 2020 Feb 27. Semin Cancer Biol. 2020. PMID: 32113998 Review.
Cited by
-
Multi-omics reveals the role of ENO1 in bladder cancer and constructs an epithelial-related prognostic model to predict prognosis and efficacy.Sci Rep. 2024 Jan 25;14(1):2189. doi: 10.1038/s41598-024-52573-8. Sci Rep. 2024. PMID: 38273010 Free PMC article.
-
CDCA3-MYC positive feedback loop promotes bladder cancer progression via ENO1-mediated glycolysis.J Exp Clin Cancer Res. 2025 Feb 20;44(1):63. doi: 10.1186/s13046-025-03325-7. J Exp Clin Cancer Res. 2025. PMID: 39980052 Free PMC article.
-
CCDC25 suppresses clear cell renal cell carcinoma progression by LATS1/YAP-mediated regulation of the hippo pathway.Cancer Cell Int. 2024 Apr 3;24(1):124. doi: 10.1186/s12935-024-03318-0. Cancer Cell Int. 2024. PMID: 38570766 Free PMC article.
-
Advances of E3 ligases in lung cancer.Biochem Biophys Rep. 2024 May 27;38:101740. doi: 10.1016/j.bbrep.2024.101740. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38841185 Free PMC article. Review.
-
SIX4 Activation in Inflammatory Response Drives the Transformation of Colorectal Epithelium into Inflammation and Tumor via Feedback-Enhancing Inflammatory Signaling to Induce Tumor Stemness Signaling.Int J Biol Sci. 2024 Aug 26;20(12):4618-4634. doi: 10.7150/ijbs.93411. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309424 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

