Targeting Ferroptosis as a Novel Approach to Alleviate Aortic Dissection
- PMID: 35844806
- PMCID: PMC9274489
- DOI: 10.7150/ijbs.72528
Targeting Ferroptosis as a Novel Approach to Alleviate Aortic Dissection
Abstract
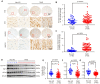
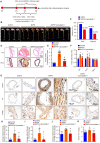
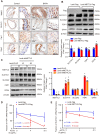
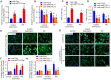
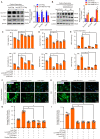
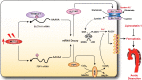
A variety of programmed cell death types have been shown to participate in the loss of smooth muscle cells (SMCs) during the development of aortic dissection (AD), but it is still largely unclear whether ferroptosis is involved in the development of AD. In the present study, we found that the expression of key ferroptosis regulatory proteins, solute carrier family 7 member 11 (SLC7A11), ferroptosis suppressor protein 1 (FSP1) and glutathione peroxidase 4 (GPX4) were downregulated in aortas of Stanford type A AD (TAAD) patients, and liproxstatin-1, a specific inhibitor of ferroptosis, obviously abolished the β-aminopropionitrile (BAPN)-induced development and rupture of AD in mice. Furthermore, the expression of methyltransferase-like 3 (METTL3), a major methyltransferase of RNA m6A, was remarkably upregulated in the aortas of TAAD patients, and the protein levels of METTL3 were negatively correlated with SLC7A11 and FSP1 levels in human aortas. Overexpression of METTL3 in human aortic SMCs (HASMCs) inhibited, while METTL3 knockdown promoted SLC7A11 and FSP1 expression. More importantly, overexpression of METTL3 facilitated imidazole ketone erastin- and cystine deprivation-induced ferroptosis, while knockdown of METTL3 repressed ferroptosis of HASMCs. Overexpression of either SLC7A11 or FSP1 largely abrogated the effect of METTL3 on HASMC ferroptosis. Therefore, we have revealed that ferroptosis is a critical cause of AD in both humans and mice and that METTL3 promotes ferroptosis of HASMCs by inhibiting the expression of SLC7A11 and FSP1. Thus, targeting ferroptosis or m6A RNA methylation is a potential novel strategy for the treatment of AD.
Keywords: Aortic dissection; FSP1/AIFM2; Ferroptosis; Liproxstatin-1; METTL3; SLC7A11.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H. et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

