Sublingual Sufentanil vs. Intravenous Fentanyl for the Treatment of Acute Postoperative Pain in the Ambulatory Surgery Center: A Randomized Clinical Trial
- PMID: 35844809
- PMCID: PMC9286986
- DOI: 10.1155/2022/5237877
Sublingual Sufentanil vs. Intravenous Fentanyl for the Treatment of Acute Postoperative Pain in the Ambulatory Surgery Center: A Randomized Clinical Trial
Abstract
Objectives: Sublingual sufentanil is a novel opioid medication to treat moderate to severe pain postoperatively. This study's aim was to determine if a single dose of a sublingual sufentanil tablet (SST) is as efficacious as a single dose of intravenous (IV) fentanyl in readiness to discharge from ambulatory surgery.
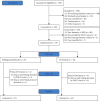
Methods: This was a two-arm, parallel group, randomized prospective outcomes study conducted at a single, free-standing ambulatory surgery center. Patients aged 18-80 undergoing general anesthesia who developed a postoperative pain score of ≥ 4 were enrolled and randomized to receive either 30 mcg SST or 50 mcg IV fentanyl. After their initial randomized dose, rescue IV fentanyl followed by oral oxycodone if needed. Recovery length of stay from arrival in the postanesthesia care unit until readiness to discharge criteria was met based on phase 2 discharge criteria.
Results: 75 patients were analyzed. Readiness to discharge from the recovery room was not significantly different between either group (IV fentanyl median 65 minutes; IQR 56-89; SST 73 min, IQR 58-89; p=0.903). There was no significant difference in the amount of morphine equivalents (MME) of rescue opioids needed (IV fentanyl median rescue MME of 22.5, IQR 13.1-23.4; SST median rescue MME of 15.0, IQR 7.5-30.0; p=0.742). The change in pain from PACU initially, and on discharge was not significantly different (IV fentanyl initial pain minus pain on discharge median 3, IQR 2-4; SST initial pain minus pain on discharge median 4, IQR 2-5.5; p=0.079). There was no difference in the six-item screener and the Overall Benefit of Analgesic Survey Score. Discussion. In conclusion, patients who received a sublingual sufentanil 30 mcg tablet had no significant differences in PACU length of stay or rescue analgesic usage when compared to intravenous fentanyl 50 mcg.
Copyright © 2022 Aaron Berg et al.
Conflict of interest statement
JH was a speaker and consultant, and has received research funds from Pacira Bioscience, he is a consultant and owns stock with Insitu Biologics, is a consultant and speaker for AcelRx, a consultant for Worrell, received research funds from Avanos, is a consultant for Atricure, and was a consultant for Johnson and Johnson. AB is a consultant for Pacira Bioscience. None of the other authors have any conflicts of interest.
References
-
- Hutchins J. L., Leiman D., Rafique Z., Di Donato K. P., Palmer P. P. Pooled dosing and efficacy analysis of the sufentanil sublingual tablet 30 mcg across demographic subgroups for the management of moderate-to-severe acute pain. Journal of Peri Anesthesia Nursing . 2020;35(1):22–28. doi: 10.1016/j.jopan.2019.08.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous