Engineering of a trispecific tumor-targeted immunotherapy incorporating 4-1BB co-stimulation and PD-L1 blockade
- PMID: 35844969
- PMCID: PMC9278964
- DOI: 10.1080/2162402X.2021.2004661
Engineering of a trispecific tumor-targeted immunotherapy incorporating 4-1BB co-stimulation and PD-L1 blockade
Abstract
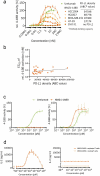
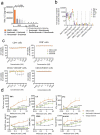
Co-stimulatory 4-1BB receptors on tumor-infiltrating T cells are a compelling target for overcoming resistance to immune checkpoint inhibitors, but initial clinical studies of 4-1BB agonist mAbs were accompanied by liver toxicity. We sought to engineer a tri-specific antibody-based molecule that stimulates intratumoral 4-1BB and blocks PD-L1/PD-1 signaling without systemic toxicity and with clinically favorable pharmacokinetics. Recombinant fusion proteins were constructed using scMATCH3 technology and humanized antibody single-chain variable fragments against PD-L1, 4-1BB, and human serum albumin. Paratope affinities were optimized using single amino acid substitutions, leading to design of the drug candidate NM21-1480. Multiple in vitro experiments evaluated pharmacodynamic properties of NM21-1480, and syngeneic mouse tumor models assessed antitumor efficacy and safety of murine analogues. A GLP multiple-dose toxicology study evaluated its safety in non-human primates. NM21-1480 inhibited PD-L1/PD-1 signaling with a potency similar to avelumab, and it potently stimulated 4-1BB signaling only in the presence of PD-L1, while exhibiting an EC50 that was largely independent of PD-L1 density. NM21-1480 exhibited high efficacy for co-activation of pre-stimulated T cells and dendritic cells. In xenograft models in syngeneic mice, NM21-1480 induced tumor regression and tumor infiltration of T cells without causing systemic T-cell activation. A GLP toxicology study revealed no evidence of liver toxicity at doses up to 140 mg/kg, and pharmacokinetic studies in non-human primates suggested a plasma half-life in humans of up to 2 weeks. NM21-1480 has the potential to overcome checkpoint resistance by co-activating tumor-infiltrating lymphocytes without liver toxicity.
Keywords: Immune checkpoint inhibitor; T-cell stimulation; antibody fragment; cancer immunotherapy; fusion protein; non-human primate; trispecific antibodies; xenograft model.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
All authors are employees of Numab Therapeutics AG. Research and manuscript preparation supported by Numab Therapeutics AG.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
