Left Atrial Cardiomyopathy - A Challenging Diagnosis
- PMID: 35845077
- PMCID: PMC9280085
- DOI: 10.3389/fcvm.2022.942385
Left Atrial Cardiomyopathy - A Challenging Diagnosis
Abstract
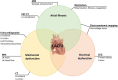
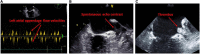
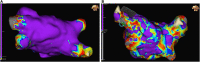
Left atrial cardiomyopathy (LACM) has been an ongoing focus of research for several years. There is evidence that LACM is responsible for atrial fibrillation and embolic strokes of undetermined sources. Therefore, the correct diagnosis of LACM is of clinical importance. Various techniques, including electrocardiography, echocardiography, cardiac magnetic resonance imaging, computed tomography, electroanatomic mapping, genetic testing, and biomarkers, can both identify and quantify structural, mechanical as well as electrical dysfunction in the atria. However, the question arises whether these techniques can reliably diagnose LACM. Because of its heterogeneity, clinical diagnosis is challenging. To date, there are no recommendations for standardized diagnosis of suspected LACM. However, standardization could help to classify LACM more precisely and derive therapeutic directions to improve individual patient management. In addition, uniform diagnostic criteria for LACM could be important for future studies. Combining several parameters and relating them seems beneficial to approach the diagnosis of LACM. This review provides an overview of the current evidence regarding the diagnosis of LACM, in which several potential parameters are discussed and, consequently, a proposal for a diagnostic algorithm is presented.
Keywords: atrial cardiomyopathy; atrial fibrillation; diagnosis; diagnostic algorithm; embolic stroke of undetermined source.
Copyright © 2022 Kreimer and Gotzmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Guichard J-B, Nattel S. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol. (2017) 70:756–65. - PubMed
-
- Peng W, Li M, Li H, Tang K, Zhuang J, Zhang J, et al. Dysfunction of myosin light-chain 4 (MYL4) leads to heritable atrial cardiomyopathy with electrical, contractile, and structural components: evidence from genetically-engineered rats. J Am Heart Assoc. (2017) 6:e007030. 10.1161/JAHA.117.007030 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

