Characterization of Early Inflammatory Events Leading to Provoked Vulvodynia Development in Rats
- PMID: 35845089
- PMCID: PMC9286136
- DOI: 10.2147/JIR.S367193
Characterization of Early Inflammatory Events Leading to Provoked Vulvodynia Development in Rats
Abstract
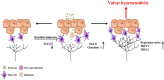
Background: Provoked vulvodynia (PV) is the main cause of vulvar pain and dyspareunia. The etiology of PV has not yet been elucidated. However, PV is associated with a history of recurrent inflammation, and its often accompanied by increases in the numbers of mast cells (MCs) and sensory hyperinnervation in the vulva. Therefore, this study aimed to examine the role of MCs and the early inflammatory events in the development of chronic vulvar pain in a rat model of PV.
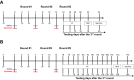
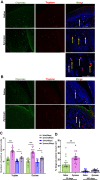
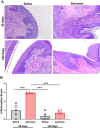
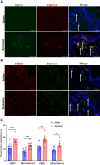
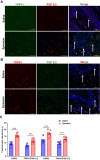
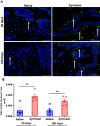
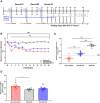
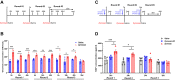
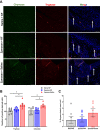
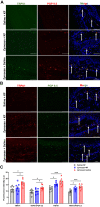
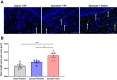
Methods: Mechanical and thermal vulvar sensitivity was measured for 5 months following zymosan vulvar challenges. Vulvar changes in glutamate and nerve growth factor (NGF) were analyzed using ELISA. Immunofluorescence (IF) staining of the vulvar section after 20, 81, and 160 days of the zymosan challenge were performed to test MCs accumulation, hyperinnervation, and expression of pain channels (transient receptor potential vanilloid/ankyrin-1-TRPV1 & TRPA1) in vulvar neurons. Changes in the development of vulvar pain were evaluated following the administration of the MCs stabilizer ketotifen fumarate (KF) during zymosan vulvar challenges.
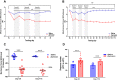
Results: Zymosan-challenged rats developed significant mechanical and thermal vulvar sensitivity that persisted for over 160 days after the zymosan challenge. During inflammation, increased local concentrations of NGF and glutamate and a robust increase in MCs degranulation were observed in zymosan-challenged rats. In addition, zymosan-challenged rats displayed sensory hyperinnervation and an increase in the expression of TRPV1 and TRPA1. Treatment with KF attenuated the upregulated level of NGF during inflammation, modulated the neuronal modifications, reduced MCs accumulation, and enhanced mechanical hypersensitivity after repeated inflammation challenges.
Conclusion: The present findings suggest that vulvar hypersensitivity is mediated by MCs accumulation, nerve growth, and neuromodulation of TRPV1 and TRPA1. Hence, KF treatment during the critical period of inflammation contributes to preventing chronic vulvar pain development.
Keywords: glutamate; hyperinnervation; inflammation; mast cell; nerve growth factor; provoked vulvodynia.
© 2022 Awad-Igbaria et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in relation to this work.
Figures
Similar articles
-
Inflammation-induced mast cell-derived nerve growth factor: a key player in chronic vulvar pain?Brain. 2025 Jan 7;148(1):331-346. doi: 10.1093/brain/awae228. Brain. 2025. PMID: 39001871
-
The Involvement of Glutamate-mGluR5 Signaling in the Development of Vulvar Hypersensitivity.Int J Mol Sci. 2025 Jan 9;26(2):523. doi: 10.3390/ijms26020523. Int J Mol Sci. 2025. PMID: 39859236 Free PMC article.
-
Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia.Sci Transl Med. 2011 Sep 21;3(101):101ra91. doi: 10.1126/scitranslmed.3002613. Sci Transl Med. 2011. PMID: 21937756 Free PMC article.
-
Localized Provoked Vulvodynia-An Ignored Vulvar Pain Syndrome.Front Cell Infect Microbiol. 2021 Jun 17;11:678961. doi: 10.3389/fcimb.2021.678961. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34222047 Free PMC article. Review.
-
Innervation of the human vulvar vestibule: A comprehensive review.Clin Anat. 2023 Jan;36(1):18-27. doi: 10.1002/ca.23966. Epub 2022 Oct 19. Clin Anat. 2023. PMID: 36216779 Review.
Cited by
-
Immune mechanisms in vulvodynia: key roles for mast cells and fibroblasts.Front Cell Infect Microbiol. 2023 Jun 8;13:1215380. doi: 10.3389/fcimb.2023.1215380. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37360527 Free PMC article. Review.
-
Exploring Localized Provoked Vulvodynia: Insights from Animal Model Research.Int J Mol Sci. 2024 Apr 11;25(8):4261. doi: 10.3390/ijms25084261. Int J Mol Sci. 2024. PMID: 38673846 Free PMC article.
-
Inflammation, lipid dysregulation, and transient receptor potential cation channel subfamily V member 4 signaling perpetuate chronic vulvar pain.Pain. 2024 Apr 1;165(4):820-837. doi: 10.1097/j.pain.0000000000003088. Epub 2023 Oct 26. Pain. 2024. PMID: 37889581 Free PMC article.
-
HBO treatment enhances motor function and modulates pain development after sciatic nerve injury via protection the mitochondrial function.J Transl Med. 2023 Aug 15;21(1):545. doi: 10.1186/s12967-023-04414-x. J Transl Med. 2023. PMID: 37582750 Free PMC article.
-
Inflammation, lipids, and pain in vulvar disease.Pharmacol Ther. 2023 Aug;248:108467. doi: 10.1016/j.pharmthera.2023.108467. Epub 2023 Jun 5. Pharmacol Ther. 2023. PMID: 37285943 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

