ABO subgroup incompatibility with severe hemolysis after consecutive allogeneic stem cell transplantations
- PMID: 35845280
- PMCID: PMC9175969
- DOI: 10.1002/jha2.190
ABO subgroup incompatibility with severe hemolysis after consecutive allogeneic stem cell transplantations
Abstract
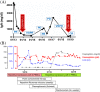
Allogeneic hematopoietic stem cell transplantations (HSCTs) represent a curative strategy for treating hematologic malignancies yet bear dangerous and frequently life-threatening complications including the development of graft-versus-host disease. Here, we present a case of a patient that suffered from relapsed/refractory multiple myeloma, a hematologic neoplasm characterized by clonal proliferation of malignant plasma cells in the bone marrow. During the course of his disease, the patient underwent consecutive allogeneic HSCTs, during which he developed a clinical meaningful and hitherto unreported ABO subgroup incompatibility, leading to persistent hemolysis. Testing for ABO subgroups during donor selection, especially after consecutive allogeneic HSCTs, may therefore aid to prevent these complications.
Keywords: A1/A2 subtyping of blood group A; ABO incompatibility; allogeneic hematopoietic stem cell transplantation; hemolysis.
© 2021 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Mookerjee A, Gupta R, Jasrotia S, Sahoo R, Kumar R, Thulkar S, et al. Bortezomib, lenalidomide and low‐dose dexamethasone (VRD) versus lenalidomide and low‐dose dexamethasone (Ld) for newly‐diagnosed multiple myeloma‐ a randomized phase III study. Blood. 2017;130:906.
-
- Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment‐refractory multiple myeloma (SIRIUS): an open‐label, randomised, phase 2 trial. Lancet. 2016;387:1551–60. - PubMed
-
- Garvey B. Rituximab in the treatment of autoimmune haematological disorders. Br. J. Haematol. 2008;141:149–69. - PubMed
-
- Barcellini W, Zanella A. Rituximab therapy for autoimmune haematological diseases. Eur J Intern Med. 2011;22:220–9. - PubMed
-
- Sandilya V. Therapeutic plasma exchange (TPE, plasmapheresis) for treatment of refractory autoimmune hemolytic anemia (AIHA). Blood. 2008;112:5380–0.
Publication types
LinkOut - more resources
Full Text Sources
