Lopinavir-menthol co-crystals for enhanced dissolution rate and intestinal absorption
- PMID: 35845293
- PMCID: PMC9272570
- DOI: 10.1016/j.jddst.2022.103587
Lopinavir-menthol co-crystals for enhanced dissolution rate and intestinal absorption
Abstract
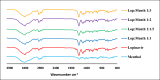
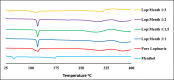
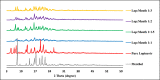
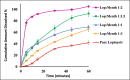
Lopinavir is an antiretroviral, antiparasitic agent and recently utilized in treatment of COVID-19. Unfortunately, lopinavir exhibited poor oral bioavailability due to poor dissolution, extensive pre-systemic metabolism, and significant P-glycoprotein intestinal efflux. Accordingly, the aim was to enhance dissolution rate and intestinal absorption of lopinavir. This employed co-processing with menthol which is believed to modify crystalline structures and inhibit intestinal efflux. Lopinavir was mixed with menthol at different molar ratios before ethanol assisted kneading. Formulations were evaluated using FTIR spectroscopy, differential scanning calorimetry (DSC), X-ray powder diffraction (XRD) and dissolution studies. Optimum ratio was utilized to assess lopinavir intestinal permeability. This employed in situ rabbit intestinal perfusion technique. FTIR, DSC and XRD indicated formation of lopinavir-menthol co-crystals at optimum molar ratio of 1:2. Additional menthol underwent phase separation due to possible self-association. Co-crystallization significantly enhanced lopinavir dissolution rate compared with pure drug to increase the dissolution efficiency from 24.96% in case of unprocessed lopinavir to 91.43% in optimum formulation. Lopinavir showed incomplete absorption from duodenum and jejuno-iliac segments with lower absorptive clearance from jejuno-ileum reflecting P-gp efflux. Co-perfusion with menthol increased lopinavir intestinal permeability. The study introduced menthol as co-crystal co-former for enhanced dissolution and augmented intestinal absorption of lopinavir.
Keywords: Co-crystal; Dissolution rate; In situ; Intestinal permeability; Lopinavir; Menthol.
© 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Preparation of orodispersible tablets of bosentan using xylitol and menthol as dissolution enhancers.Sci Rep. 2024 May 9;14(1):10680. doi: 10.1038/s41598-024-60494-9. Sci Rep. 2024. PMID: 38724608 Free PMC article.
-
Menthol augmented niosomes for enhanced intestinal absorption of lopinavir.Pharm Dev Technol. 2022 Nov;27(9):956-964. doi: 10.1080/10837450.2022.2136195. Epub 2022 Oct 21. Pharm Dev Technol. 2022. PMID: 36227222
-
Xylitol as a potential co-crystal co-former for enhancing dissolution rate of felodipine: preparation and evaluation of sublingual tablets.Pharm Dev Technol. 2018 Jun;23(5):454-463. doi: 10.1080/10837450.2016.1242625. Epub 2016 Nov 3. Pharm Dev Technol. 2018. PMID: 27681386
-
Sucralose as co-crystal co-former for hydrochlorothiazide: development of oral disintegrating tablets.Drug Dev Ind Pharm. 2016 Aug;42(8):1225-33. doi: 10.3109/03639045.2015.1118495. Epub 2015 Dec 7. Drug Dev Ind Pharm. 2016. PMID: 26555927
-
Effect of binary and ternary solid dispersions on the in vitro dissolution and in-situ rabbit intestinal absorption of gliclazide.Pak J Pharm Sci. 2011 Oct;24(4):459-68. Pak J Pharm Sci. 2011. PMID: 21959805
Cited by
-
New Lidocaine-Based Pharmaceutical Cocrystals: Preparation, Characterization, and Influence of the Racemic vs. Enantiopure Coformer on the Physico-Chemical Properties.Pharmaceutics. 2023 Mar 29;15(4):1102. doi: 10.3390/pharmaceutics15041102. Pharmaceutics. 2023. PMID: 37111588 Free PMC article.
-
Bilosomes and Niosomes for Enhanced Intestinal Absorption and In Vivo Efficacy of Cytarabine in Treatment of Acute Myeloid Leukemia.Pharmaceuticals (Basel). 2024 Nov 23;17(12):1572. doi: 10.3390/ph17121572. Pharmaceuticals (Basel). 2024. PMID: 39770414 Free PMC article.
-
Glyceroniosomes for enhanced intestinal absorption of hydrochlorothiazide and lisinopril in their fixed dose combination.Sci Rep. 2024 Oct 18;14(1):24499. doi: 10.1038/s41598-024-74986-1. Sci Rep. 2024. PMID: 39424875 Free PMC article.
-
Preparation of orodispersible tablets of bosentan using xylitol and menthol as dissolution enhancers.Sci Rep. 2024 May 9;14(1):10680. doi: 10.1038/s41598-024-60494-9. Sci Rep. 2024. PMID: 38724608 Free PMC article.
-
Electrospun Amorphous Solid Dispersions with Lopinavir and Ritonavir for Improved Solubility and Dissolution Rate.Nanomaterials (Basel). 2024 Sep 28;14(19):1569. doi: 10.3390/nano14191569. Nanomaterials (Basel). 2024. PMID: 39404296 Free PMC article.
References
-
- Khan A.A., Mudassir J., Akhtar S., Murugaiyah V., Darwis Y. Freeze-dried lopinavir-loaded nanostructured lipid carriers for enhanced cellular uptake and bioavailability: statistical optimization, in vitro and in vivo evaluations. Pharmaceutics. 2019;11:97. doi: 10.3390/pharmaceutics11020097. - DOI - PMC - PubMed
-
- Chiu M.L., Liang W.M., Li J.P., Cheng C.F., Chiou J.S., Ho M.W., Wu Y.C., Lin T.H., Liao C.C., Huang S.M., Tsai F.J. Dosage, and adherence of antiretroviral therapy and risk of osteoporosis in patients with human immunodeficiency virus infection in taiwan: a nested case-control study. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.631480. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

