Risk of further decompensation/mortality in patients with cirrhosis and ascites as the first single decompensation event
- PMID: 35845294
- PMCID: PMC9284386
- DOI: 10.1016/j.jhepr.2022.100513
Risk of further decompensation/mortality in patients with cirrhosis and ascites as the first single decompensation event
Erratum in
-
Erratum Regarding Previously Published Articles.JHEP Rep. 2024 May 18;6(6):101096. doi: 10.1016/j.jhepr.2024.101096. eCollection 2024 Jun. JHEP Rep. 2024. PMID: 38978771 Free PMC article.
Abstract
Background & aims: Although ascites is the most frequent first decompensating event in cirrhosis, the clinical course after ascites as the single index decompensation is not well defined. The aim of this multicentre study was thus to systematically investigate the incidence and type of further decompensation after ascites as the first decompensating event and to assess risk factors for mortality.
Methods: A total of 622 patients with cirrhosis presenting with grade 2/3 ascites as the single index decompensating event at 2 university hospitals (Padova and Vienna) between 2003 and 2021 were included. Events of further decompensation, liver transplantation, and death were recorded.
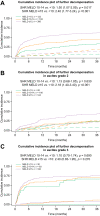
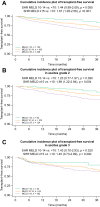
Results: The mean age was 57 ± 11 years, and most patients were male (n = 423, 68%) with alcohol-related (n = 366, 59%) and viral (n = 200,32%) liver disease as the main aetiologies. In total, 323 (52%) patients presented with grade 2 and 299 (48%) with grade 3 ascites. The median Child-Pugh score at presentation was 8 (IQR 7-9), and the mean model for end-stage liver disease (MELD) was 15 ± 6. During a median follow-up period of 49 months, 350 (56%) patients experienced further decompensation: refractory ascites (n = 130, 21%), hepatic encephalopathy (n = 112, 18%), spontaneous bacterial peritonitis (n = 32, 5%), hepatorenal syndrome-acute kidney injury (n = 29, 5%). Variceal bleeding as an isolated further decompensation event was rare (n = 18, 3%), whereas non-bleeding further decompensation (n = 161, 26%) and ≥2 concomitant further decompensation events (n = 171, 27%) were frequent. Transjugular intrahepatic portosystemic shunt was used in only 81 (13%) patients. In patients presenting with grade 2 ascites, MELD ≥15 indicated a considerable risk for further decompensation (subdistribution hazard ratio [SHR] 2.18; p <0.001; 1-year incidences: <10: 10% vs. 10-14: 13% vs. ≥15: 28%) and of mortality (SHR 1.89; p = 0.004; 1-year incidences: <10: 3% vs. 10-14: 6% vs. ≥15: 14%). Importantly, mortality was similarly high throughout MELD strata in grade 3 ascites (p = n.s. for different MELD strata; 1-year incidences: <10: 14% vs. 10-14: 15% vs. ≥15: 20%).
Conclusions: Further decompensation is frequent in patients with ascites as a single index decompensation event and only rarely owing to bleeding. Although patients with grade 2 ascites and MELD <15 seem to have a favourable prognosis, those with grade 3 ascites are at a high risk of mortality across all MELD strata.
Lay summary: Decompensation (the development of symptoms as a result of worsening liver function) marks a turning point in the disease course for patients with cirrhosis. Ascites (i.e. , the accumulation of fluid in the abdomen) is the most common first decompensating event, yet little is known about the clinical course of patients who develop ascites as a single first decompensating event. Herein, we show that the severity of ascites is associated with mortality and that in patients with moderate ascites, the widely used prognostic MELD score can predict patient outcomes.
Keywords: ACLF, acute-on-chronic liver failure; Acute kidney injury; Baveno; CI, confidence interval; CPS, Child–Pugh score; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; HRS-AKI, hepatorenal syndrome–acute kidney injury; Hepatic decompensation; ICA, International Club of Ascites; INR, international normalised ratio; IQR, interquartile range; LT, liver transplantation; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PVT, portal vein thrombosis; Portal hypertension; RA, refractory ascites; SBP, spontaneous bacterial peritonitis; SHR, subdistribution hazard ratio; Spontaneous bacterial peritonitis; TFS, transplant-free survival; TIPS, transjugular intrahepatic portosystemic shunt; UNOS MELD (2016), United Network for Organ Sharing model for end-stage liver disease (2016); VB, variceal bleeding; aSHR, adjusted subdistribution hazard ratio.
© 2022 The Author(s).
Conflict of interest statement
The authors have nothing to disclose regarding the work under consideration for publication. The following authors disclose conflicts of interests outside the submitted work: LB, MTo, GS, VC, LH, SI, MJ, BSH, CGC, AA, AB, and SP have nothing to disclose. DB received travel support from AbbVie and Gilead and speaker fees from AbbVie and Siemens, as well as grant support form Gilead and Siemens. MTr received grant support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and UltraGenyx; honouraria for consulting from Albireo, Boehringer Ingelheim, BiomX, Falk, Genfit, Gilead, Intercept, Janssen, MSD, Novartis, Phenex, Pliant, Regulus, and Shire; speaker fees from Bristol-Myers Squibb, Falk, Gilead, Intercept, and MSD; and travel support from AbbVie, Falk, Gilead, and Intercept. MM served as a speaker and/or consultant and/or advisory board member for AbbVie, Collective Acumen, Gilead, and W.L. Gore & Associates and received travel support from AbbVie and Gilead. TR received grant support from AbbVie, Boehringer-Ingelheim, Gilead, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, Siemens, and W.L. Gore & Associates; speaking honoraria from AbbVie, Gilead, Gore, Intercept, Roche, and MSD; consulting/advisory board fees from AbbVie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, and Siemens; and travel support from AbbVie, Boehringer-Ingelheim, Gilead, and Roche. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- D'Amico G., Pasta L., Morabito A., D'Amico M., Caltagirone M., Malizia G., et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180–1193. - PubMed
-
- Ginés P., Quintero E., Arroyo V., Terés J., Bruguera M., Rimola A., et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. - PubMed
-
- Jepsen P., Ott P., Andersen P.K., Sørensen H.T., Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. - PubMed
-
- Solà E., Watson H., Graupera I., Turón F., Barreto R., Rodríguez E., et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol. 2012;57:1199–1206. - PubMed
-
- Campbell M.S., Brensinger C.M., Sanyal A.J., Gennings C., Wong F., Kowdley K.V., et al. Quality of life in refractory ascites: transjugular intrahepatic portal-systemic shunting versus medical therapy. Hepatology. 2005;42:635–640. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

