Effects of Different Lengths of a Nucleic Acid Binding Region and Bound Nucleic Acids on the Phase Behavior and Purification Process of HBcAg Virus-Like Particles
- PMID: 35845397
- PMCID: PMC9283707
- DOI: 10.3389/fbioe.2022.929243
Effects of Different Lengths of a Nucleic Acid Binding Region and Bound Nucleic Acids on the Phase Behavior and Purification Process of HBcAg Virus-Like Particles
Abstract
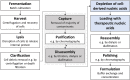
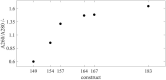
Virus-like particles (VLPs) are macromolecular structures with great potential as vehicles for the targeted administration of functional molecules. Loaded with nucleic acids, VLPs are a promising approach for nanocarriers needed for gene therapy. There is broad knowledge of the manufacturing of the truncated wild-type lacking a nucleic acid binding region, which is mainly being investigated for vaccine applications. Whereas for their potential application as a nanocarrier for gene therapy, hepatitis B core antigen (HBcAg) VLPs with a nucleic acid binding region for efficient cargo-loading are being investigated. VLP structure, loading, and phase behavior are of central importance to their therapeutic efficacy and thereby considerably affecting the production process. Therefore, HBcAg VLPs with different lengths of the nucleic acid binding region were produced in E. coli. VLP attributes such as size, zeta potential, and loading with host cell-derived nucleic acids were evaluated. Capsid's size and zeta potential of the VLP constructs did not differ remarkably, whereas the analysis of the loading with host cell-derived nucleic acids revealed strong differences in the binding of host cell-derived nucleic acids dependent on the length of the binding region of the constructs, with a non-linear correlation but a two-zone behavior. Moreover, the phase behavior and purification process of the HBcAg VLPs as a function of the liquid phase conditions and the presence of host cell-derived nucleic acids were investigated. Selective VLP precipitation using ammonium sulfate was scarcely affected by the encapsulated nucleic acids. However, the disassembly reaction, which is crucial for structure homogeneity, separation of encapsulated impurities, and effective loading of the VLPs with therapeutic nucleic acids, was affected both by the studied liquid phase conditions, varying pH and concentration of reducing agents, and the different VLP constructs and amount of bound nucleic acids, respectively. Thereby, capsid-stabilizing effects of the bound nucleic acids and capsid-destabilizing effects of the nucleic acid binding region were observed, following the two-zone behavior of the construct's loading, and a resulting correlation between the capsid stability and disassembly yields could be derived.
Keywords: capsid stability; disassembly; downstream processing; gene therapy; nanocarrier (nanoparticle); nucleic acid binding; phase behavior; virus-like particles.
Copyright © 2022 Valentic, Müller and Hubbuch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

