The use of Esketamine in CT-guided percutaneous liver tumor ablation reduces the consumption of remifentanil: a randomized, controlled, double-blind trial
- PMID: 35845514
- PMCID: PMC9279786
- DOI: 10.21037/atm-22-2756
The use of Esketamine in CT-guided percutaneous liver tumor ablation reduces the consumption of remifentanil: a randomized, controlled, double-blind trial
Abstract
Background: In the anesthesia management of percutaneous liver tumor ablation, the requirement of analgesia is very strict. Currently, intravenous anesthesia is commonly used, such as remifentanil combined with sedative drugs. However, the pain relief is not instantaneous after increasing the dosage of remifentanil. Esketamine, a medium- or long-term analgesic drug, does not inhibit respiration to maintain patient comfort during the ablation and reduces the consumption of remifentanil. Therefore, this experiment was designed to investigate the potential of combinational therapy and the most appropriate dose of esketamine.
Methods: A total of 120 patients were randomly divided into three groups by SPSS. The regular anesthesia model included dexmedetomidine 0.5 µg/kg, intravenous glucose tolerance test, remifentanil continuous infusion, flurbiprofen 50 mg, i.v., palonosetron 0.225 mg, i.v., and 1% lidocaine for local anesthesia. Group A was the regular control group, only using the regular model; Group B also received with 0.1 mg/kg esketamine, i.v.; and Group C also received 0.2 mg/kg esketamine, i.v.. The whole experiment was double-blind.
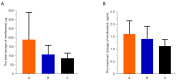
Results: From December 2020 to March 2021, 120 patients were randomized in total, and 108 were included in the analysis: 36, 37, 35 were allocated to Group A, Group B, and Group C, respectively. The total dosage of remifentanil in Group A, Group B, Group C was 179.38±123.37, 120.31±57.96 and 115.91±62.42 µg, respectively. We found the total dosage of remifentanil in Group B and Group C were significantly decreased in comparison to that of Group A (P=0.004, P=0.003, respectively). The maximum dosage of remifentanil in Group A, Group B, and Group C was 1.76±0.62, 1.37±0.47, and 1.33±0.56 ng/mL, respectively. The maximum dosage of remifentanil in Group B and Group C were significantly decreased in comparison to that of Group A (P=0.003, P=0.001, respectively). The incidence of severe pain during the ablation in Group B was significantly lower than that in Group A (3 vs. 12, P<0.05).
Conclusions: The use of esketamine can reduce the dosage of opioids for liver tumor ablation and reduce the occurrence of severe pain. We found that 0.1 mg/kg esketamine, i.v. is the most suitable dose for liver tumor ablation.
Trial registration: Chinese Clinical Trial Registry ChiCTR2100049152.
Keywords: Esketamine; ablation; analgesia; liver tumor; sedation.
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2756/coif). The authors have no conflicts of interest to declare.
Figures




References
-
- Sabo B, Dodd GD, 3rd, Halff GA, et al. Anesthetic considerations in patients undergoing percutaneous radiofrequency interstitial tissue ablation. AANA J 1999;67:467-8. - PubMed
-
- Amornyotin S, Jirachaipitak S, Wangnatip S. Anesthetic management for radiofrequency ablation in patients with hepatocellular carcinoma in a developing country. J Anesth Crit Care Open Access 2015;3:00086.
LinkOut - more resources
Full Text Sources
