If At First You Don't Succeed, Trikafta Again
- PMID: 35845559
- PMCID: PMC9268110
- DOI: 10.5863/1551-6776-27.5.467
If At First You Don't Succeed, Trikafta Again
Abstract
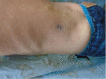
Adverse reactions, including severe cutaneous reactions, to cystic fibrosis transmembrane conductance regulator (CFTR) modulators have been described in the literature. Herein we present a drug eruption in response to elexacaftor/tezcaftor/ivacaftor (brand name, Trikafta) in a 7-year-old male with cystic fibrosis, followed by desensitization and successful continuation. A review of the literature outlining similar cases is provided. Attempting to mitigate and manage drug reactions to CFTR modulators is essential because they represent vital and irreplaceable therapies for individuals with cystic fibrosis (CF).
Keywords: CFTR modulator; cystic fibrosis; desensitizat urticaria; elexacaftor; ivacaftor; tezacaftor.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: membership@pediatricpharmacy.org 2022.
Conflict of interest statement
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript. The authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
References
-
- Elborn JS. Cystic fibrosis. Lancet . 2016;388(10059):2519–2531. - PubMed
-
- Griese M, Costa S, Linnemann RW et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for 24 weeks or longer in people with cystic fibrosis and one or more F508del alleles: interim results of an open-label phase 3 clinical trial. Am J Respir Crit Care Med . 2021;203(3):381–385. - PMC - PubMed
LinkOut - more resources
Full Text Sources