Rechallenge of Elexacaftor/Tezacaftor/Ivacaftor After Skin Rash in Two Pediatric Patients
- PMID: 35845562
- PMCID: PMC9268115
- DOI: 10.5863/1551-6776-27.5.463
Rechallenge of Elexacaftor/Tezacaftor/Ivacaftor After Skin Rash in Two Pediatric Patients
Abstract
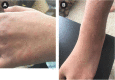
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have revolutionized care for patients with cystic fibrosis (CF). The triple combination product elexacaftor/tezacaftor/ivacaftor is a highly effective CFTR modulator that is generally well tolerated. However, in clinical trials of pediatric and adult patients, 4% to 12% developed rash after initiation of therapy. Few reports have described approaches to management of this adverse effect. In this report, we describe 2 children with CF who developed a pruritic, maculopapular rash after initiating elexacaftor/tezacaftor/ivacaftor. These patients were successfully rechallenged after rash resolution with a practical titration schedule.
Keywords: CFTR modulator; cystic fibrosis; rash.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: membership@pediatricpharmacy.org 2022.
Conflict of interest statement
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment gifts, and honoraria. PJM receives grant support from the Cystic Fibrosis Foundation, Eloxx, and Vertex Pharmaceuticals. Authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
Figures
Similar articles
-
Triple Therapy for Cystic Fibrosis (Elexacaftor, Tezacaftor, and Ivacaftor): Desensitization After Skin Rash.Cureus. 2023 Sep 29;15(9):e46228. doi: 10.7759/cureus.46228. eCollection 2023 Sep. Cureus. 2023. PMID: 37905296 Free PMC article.
-
Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial.Lancet Respir Med. 2022 Mar;10(3):267-277. doi: 10.1016/S2213-2600(21)00454-9. Epub 2021 Dec 20. Lancet Respir Med. 2022. PMID: 34942085 Clinical Trial.
-
Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial.Lancet. 2019 Nov 23;394(10212):1940-1948. doi: 10.1016/S0140-6736(19)32597-8. Epub 2019 Oct 31. Lancet. 2019. PMID: 31679946 Free PMC article. Clinical Trial.
-
Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy.J Pediatr Pharmacol Ther. 2020;25(3):192-197. doi: 10.5863/1551-6776-25.3.192. J Pediatr Pharmacol Ther. 2020. PMID: 32265602 Free PMC article. Review.
-
The preclinical discovery and development of the combination of ivacaftor + tezacaftor used to treat cystic fibrosis.Expert Opin Drug Discov. 2020 Aug;15(8):873-891. doi: 10.1080/17460441.2020.1750592. Epub 2020 Apr 15. Expert Opin Drug Discov. 2020. PMID: 32290721 Review.
Cited by
-
Pharmacist to the Rescue: Overcoming Obstacles for Select Patients.J Pediatr Pharmacol Ther. 2022;27(5):407-408. doi: 10.5863/1551-6776-27.5.407. Epub 2022 Jul 6. J Pediatr Pharmacol Ther. 2022. PMID: 35845564 Free PMC article. No abstract available.
-
Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis.Pharmaceuticals (Basel). 2023 Mar 8;16(3):410. doi: 10.3390/ph16030410. Pharmaceuticals (Basel). 2023. PMID: 36986509 Free PMC article. Review.
-
Recent developments in cystic fibrosis drug discovery: where are we today?Expert Opin Drug Discov. 2025 May;20(5):659-682. doi: 10.1080/17460441.2025.2490250. Epub 2025 Apr 13. Expert Opin Drug Discov. 2025. PMID: 40202089 Review.
-
Triple Therapy for Cystic Fibrosis (Elexacaftor, Tezacaftor, and Ivacaftor): Desensitization After Skin Rash.Cureus. 2023 Sep 29;15(9):e46228. doi: 10.7759/cureus.46228. eCollection 2023 Sep. Cureus. 2023. PMID: 37905296 Free PMC article.
-
Malassezia Folliculitis following Triple Therapy for Cystic Fibrosis.Medicina (Kaunas). 2022 Sep 2;58(9):1204. doi: 10.3390/medicina58091204. Medicina (Kaunas). 2022. PMID: 36143881 Free PMC article.
References
-
- Boston, MA: Vertex Pharmaceuticals Inc; Oct, 2021. TRIKAFTA (elexacaftor, tezacaftor and ivacaftor tablets) [package insert]
-
- Hu MK, Wood G, Dempsey O. “Triple therapy” (elexacaftor, tezacaftor, ivacaftor) skin rash in patients with cystic fibrosis. Postgrad Med J . 2022;98(1156):86. - PubMed
LinkOut - more resources
Full Text Sources