Silencing lncRNA 93358 Inhibits the Apoptosis of Myocardial Cells in Myocardial Infarction Rats by Inducing the Expression of SLC8A1
- PMID: 35845941
- PMCID: PMC9283055
- DOI: 10.1155/2022/1138709
Silencing lncRNA 93358 Inhibits the Apoptosis of Myocardial Cells in Myocardial Infarction Rats by Inducing the Expression of SLC8A1
Retraction in
-
Retracted: Silencing lncRNA 93358 Inhibits the Apoptosis of Myocardial Cells in Myocardial Infarction Rats by Inducing the Expression of SLC8A1.Biomed Res Int. 2023 Dec 6;2023:9812061. doi: 10.1155/2023/9812061. eCollection 2023. Biomed Res Int. 2023. PMID: 38094568 Free PMC article.
Abstract
Objective: To explore the inhibitor effects and mechanism of lncRNA 93358 against the apoptosis of myocardial cells in rats with myocardial infarction.
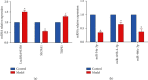
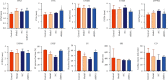
Methods: The myocardial infarction model was established in rats, which were identified by cardiac ultrasound. TTC staining was used to evaluate the degree of heart infarction, and HE staining was utilized to determine the pathological state in myocardial tissues. The apoptotic state in myocardial tissues was confirmed by TUNEL assay. lncRNA 93358 was screened out using a high-throughput sequencing which was confirmed by RT-qPCR. The interaction between miR-466c-3p and SLC8A1 was identified using the dual-luciferase reporter assay. The expression level of Bax, Bcl-2, and SLC8A1 was determined in lncRNA 93358 knockdown cells using RT-qPCR and Western blotting.
Results: Massive myocardial necrosis was observed in model rats according to the results of TTC staining, HE staining, and TUNEL assay. lncRNA 93358 and Bax were found significantly upregulated, and Bcl-2 and SLC8A1 were greatly downregulated in model rats, which were dramatically reversed by the knockdown of lncRNA 93358, accompanied by the decline area of myocardial necrosis and decreased apoptotic myocardial cells.
Conclusion: Silencing lncRNA 93358 inhibits the apoptosis of myocardial cells in rats with myocardial infarction by inducing the expression of SLC8A1.
Copyright © 2022 Jiumei Cai et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Ruddox V., Sandven I., Munkhaugen J., Skattebu J., Edvardsen T., Otterstad J. E. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. European Journal of Preventive Cardiology . 2017;24(14):1555–1566. doi: 10.1177/2047487317715769. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

