Myosin 5a in the Urinary Bladder: Localization, Splice Variant Expression, and Functional Role in Neurotransmission
- PMID: 35845995
- PMCID: PMC9284544
- DOI: 10.3389/fphys.2022.890102
Myosin 5a in the Urinary Bladder: Localization, Splice Variant Expression, and Functional Role in Neurotransmission
Abstract
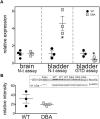
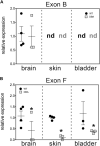
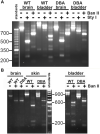
Dysregulation of neurotransmission is a feature of several prevalent lower urinary tract conditions, but the mechanisms regulating neurotransmitter release in the bladder are not completely understood. The unconventional motor protein, Myosin 5a, transports neurotransmitter-containing synaptic vesicles along actin fibers towards the varicosity membrane, tethering them at the active zone prior to reception of a nerve impulse. Our previous studies indicated that Myosin 5a is expressed and functionally relevant in the peripheral nerves of visceral organs such as the stomach and the corpora cavernosa. However, its potential role in bladder neurotransmission has not previously been investigated. The expression of Myosin 5a was examined by quantitative PCR and restriction analyses in bladders from DBA (dilute-brown-nonagouti) mice which express a Myosin 5a splicing defect and in control mice expressing the wild-type Myosin 5a allele. Functional differences in contractile responses to intramural nerve stimulation were examined by ex vivo isometric tension analysis. Data demonstrated Myosin 5a localized in cholinergic nerve fibers in the bladder and identified several Myosin 5a splice variants in the detrusor. Full-length Myosin 5a transcripts were less abundant and the expression of splice variants was altered in DBA bladders compared to control bladders. Moreover, attenuation of neurally-mediated contractile responses in DBA bladders compared to control bladders indicates that Myosin 5a facilitates excitatory neurotransmission in the bladder. Therefore, the array of Myosin 5a splice variants expressed, and the abundance of each, may be critical parameters for efficient synaptic vesicle transport and neurotransmission in the urinary bladder.
Keywords: Myosin 5a; bladder smooth muscle; myosin motor; neurotransmission; peripheral nerve; protein splice variants.
Copyright © 2022 Carew, Cristofaro, Dasari, Carey, Goyal and Sullivan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Differential Myosin 5a splice variants in innervation of pelvic organs.Front Physiol. 2023 Dec 12;14:1304537. doi: 10.3389/fphys.2023.1304537. eCollection 2023. Front Physiol. 2023. PMID: 38148903 Free PMC article.
-
Imbalance of bladder neurohomeostasis by Myosin 5a aggravates diabetic cystopathy.Mol Med. 2025 Mar 10;31(1):91. doi: 10.1186/s10020-025-01140-6. Mol Med. 2025. PMID: 40065210 Free PMC article.
-
Expression of Myosin 5a splice variants in murine stomach.Neurogastroenterol Motil. 2021 Oct;33(10):e14162. doi: 10.1111/nmo.14162. Epub 2021 May 3. Neurogastroenterol Motil. 2021. PMID: 33939222
-
Pharmacologic perspective on the physiology of the lower urinary tract.Urology. 2002 Nov;60(5 Suppl 1):13-20; discussion 20-1. doi: 10.1016/s0090-4295(02)01786-7. Urology. 2002. PMID: 12493344 Review.
-
Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles.World J Urol. 1994;12(5):274-80. doi: 10.1007/BF00191207. World J Urol. 1994. PMID: 7532516 Review.
Cited by
-
Differential Myosin 5a splice variants in innervation of pelvic organs.Front Physiol. 2023 Dec 12;14:1304537. doi: 10.3389/fphys.2023.1304537. eCollection 2023. Front Physiol. 2023. PMID: 38148903 Free PMC article.
-
Imbalance of bladder neurohomeostasis by Myosin 5a aggravates diabetic cystopathy.Mol Med. 2025 Mar 10;31(1):91. doi: 10.1186/s10020-025-01140-6. Mol Med. 2025. PMID: 40065210 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

