Measurement of neurodegeneration using a multivariate early frame amyloid PET classifier
- PMID: 35846158
- PMCID: PMC9270637
- DOI: 10.1002/trc2.12325
Measurement of neurodegeneration using a multivariate early frame amyloid PET classifier
Abstract
Introduction: Amyloid measurement provides important confirmation of pathology for Alzheimer's disease (AD) clinical trials. However, many amyloid positive (Am+) early-stage subjects do not worsen clinically during a clinical trial, and a neurodegenerative measure predictive of decline could provide critical information. Studies have shown correspondence between perfusion measured by early amyloid frames post-tracer injection and fluorodeoxyglucose (FDG) positron emission tomography (PET), but with limitations in sensitivity. Multivariate machine learning approaches may offer a more sensitive means for detection of disease related changes as we have demonstrated with FDG.
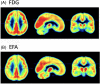
Methods: Using summed dynamic florbetapir image frames acquired during the first 6 minutes post-injection for 107 Alzheimer's Disease Neuroimaging Initiative subjects, we applied optimized machine learning to develop and test image classifiers aimed at measuring AD progression. Early frame amyloid (EFA) classification was compared to that of an independently developed FDG PET AD progression classifier by scoring the FDG scans of the same subjects at the same time point. Score distributions and correlation with clinical endpoints were compared to those obtained from FDG. Region of interest measures were compared between EFA and FDG to further understand discrimination performance.
Results: The EFA classifier produced a primary pattern similar to that of the FDG classifier whose expression correlated highly with the FDG pattern (R-squared 0.71), discriminated cognitively normal (NL) amyloid negative (Am-) subjects from all Am+ groups, and that correlated in Am+ subjects with Mini-Mental State Examination, Clinical Dementia Rating Sum of Boxes, and Alzheimer's Disease Assessment Scale-13-item Cognitive subscale (R = 0.59, 0.63, 0.73) and with subsequent 24-month changes in these measures (R = 0.67, 0.73, 0.50).
Discussion: Our results support the ability to use EFA with a multivariate machine learning-derived classifier to obtain a sensitive measure of AD-related loss in neuronal function that correlates with FDG PET in preclinical and early prodromal stages as well as in late mild cognitive impairment and dementia.
Highlights: The summed initial post-injection minutes of florbetapir positron emission tomography correlate with fluorodeoxyglucose.A machine learning classifier enabled sensitive detection of early prodromal Alzheimer's disease.Early frame amyloid (EFA) classifier scores correlate with subsequent change in Mini-Mental State Examination, Clinical Dementia Rating Sum of Boxes, and Alzheimer's Disease Assessment Scale-13-item Cognitive subscale.EFA classifier effect sizes and clinical prediction outperformed region of interest standardized uptake value ratio.EFA classification may aid in stratifying patients to assess treatment effect.
Keywords: Alzheimer's disease; EFA; amyloid; early frame amyloid; fluorodeoxyglucose; machine learning.
© 2022 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dawn C. Matthews, Ana S. Lukic, and Randolph D. Andrews are employees of ADM Diagnostics, Inc., a company that provides imaging clinical trial services and diagnostic products. Stephen C. Strother and Miles N. Wernick are senior advisors to ADM Diagnostics, Inc. Mark E. Schmidt is an employee of Janssen Pharmaceutica, NV. None of these authors have a conflict of interest regarding use of florbetapir as an amyloid PET tracer. Author disclosures are available in the supporting information.
Figures



References
-
- Siemers ER, Sundell KL, Carlson C, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12(2):110‐120. - PubMed
-
- Sevigny J, Suhy J, Chiao P, et al. Amyloid PET screening for enrichment of early‐stage Alzheimer disease clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord. 2016;30(1):1‐7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
