Characterization of real-world treatment practices and outcomes among patients with chronic lymphocytic leukemia treated in a Finnish tertiary center
- PMID: 35846189
- PMCID: PMC9176063
- DOI: 10.1002/jha2.322
Characterization of real-world treatment practices and outcomes among patients with chronic lymphocytic leukemia treated in a Finnish tertiary center
Abstract
Objectives: We conducted this retrospective study to characterize the change in chronic lymphocytic leukemia (CLL) treatment patterns between 2005 and 2019, to understand the treatment sequencing across the course of the disease, and to investigate how targeted agents and prognostic testing were implemented into the patient care.
Methods: This study included adult patients with CLL treated at the Hospital District of Southwest Finland during the study period. Data were collected from the Turku University Hospital data lake.
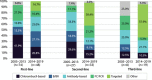
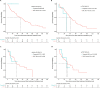
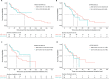
Results: In total, 122 and 60 patients received first- and second-line treatments for CLL, respectively. The shift from conventional chemoimmunotherapy to targeted treatments in recent years (2014-2019) was observed. The median overall survival times were not reached in patients treated with targeted agents compared to conventional standard treatments in first- and second-line settings and improved toward the end of the study period. Prognostic testing increased during the study follow-up and patients with unmutated immunoglobulin heavy-chain variable showed significantly poorer overall survival and time-to-next-treatment outcomes than patients with mutated immunoglobulin heavy-chain variable.
Conclusions: This real-world study implicated added value of targeted chemo-free therapies as reported in randomized clinical trials, and highlighted the necessity of prognostic testing in order to improve treatment selection and patient outcomes.
Keywords: IGHV; chemoimmunotherapy; chronic lymphocytic leukemia; survival; targeted therapy.
© 2021 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
Juha Ranti served as a consultant for Janssen, AbbVie, and AstraZeneca, on the speakers' bureau for AbbVie and received travel support from Janssen and AbbVie. Katariina Perkonoja and Tommi Kauko are present or previous employees of Auria Clinical Informatics serving both academic research and industry‐sponsored scientific studies. Heidi Loponen and Emmi I. Joensuu are employees of MedEngine Oy. Tiina M. Järvinen is an employee of Janssen‐Cilag Oy.
Figures

References
-
- Finnish Cancer Registry . Cancer statistics. https://syoparekisteri.fi/tilastot/tautitilastot/ Last Accessed September 22, 2020.
-
- Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet 2018;391:1524–37. - PubMed
-
- Hallek M, Cheson BD, Catovsky D, Caligaris‐Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018;131(25):2745–60. - PubMed
-
- Keating MJ, O'Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–88. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
