Comparative effectiveness of ciltacabtagene autoleucel in CARTITUDE-1 versus physician's choice of therapy in the Flatiron Health multiple myeloma cohort registry for the treatment of patients with relapsed or refractory multiple myeloma
- PMID: 35846215
- PMCID: PMC9175662
- DOI: 10.1002/jha2.312
Comparative effectiveness of ciltacabtagene autoleucel in CARTITUDE-1 versus physician's choice of therapy in the Flatiron Health multiple myeloma cohort registry for the treatment of patients with relapsed or refractory multiple myeloma
Abstract
Introduction: Ciltacabtagene autoleucel (cilta-cel) is a novel chimeric antigen receptor T-cell therapy that is being evaluated in the CARTITUDE-1 trial (NCT03548207) in patients with relapsed or refractory multiple myeloma (RRMM) who received as part of their previous therapy an immunomodulatory drug, proteasome inhibitor, and an anti-CD38 monoclonal antibody (i.e., triple-class exposed). Given the absence of a control arm in CARTITUDE-1, this study assessed the comparative effectiveness of cilta-cel and physician's choice of treatment (PCT) using an external real-world control arm from the Flatiron Health multiple myeloma cohort registry.
Methods: Given the availability of individual patient data for cilta-cel from CARTITUDE-1 and PCT in Flatiron, inverse probability of treatment weighting was used to adjust for unbalanced baseline covariates of prognostic significance: refractory status, cytogenetic profile, International Staging System stage, time to progression on last regimen, number of prior lines of therapy, years since diagnosis, and age. Comparative effectiveness was estimated for progression-free survival (PFS), time to next treatment (TTNT), and overall survival (OS). A range of sensitivity analyses were conducted.
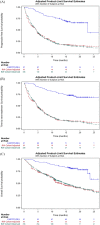
Results: Baseline characteristics were similar between the two cohorts after propensity score weighting. Patients with cilta-cel had improved PFS (HR: 0.18 [95% CI: 0.12, 0.27; p < 0.0001]), TTNT (HR: 0.15 [95% CI: 0.09, 0.22; p < 0.0001]), and OS (HR: 0.25 [95% CI: 0.13, 0.46; p < 0.0001]) versus PCT. Cilta-cel treatment benefit was robust and consistent across all sensitivity analyses.
Conclusion: Cilta-cel demonstrated significantly superior effectiveness over PCT for all outcomes, highlighting its potential as an effective therapy in patients with triple-class exposed RRMM.
Keywords: CARTITUDE‐1; Flatiron Health; ciltacabtagene autoleucel; indirect treatment comparison; relapsed or refractory multiple myeloma; triple‐class exposed.
© 2021 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
KW received honoraria from and served in a consulting or advisory role for Adaptive Biotechonlogies, Amgen, BMS, Celgene, GSK, Janssen, KaryopharmTherapeutics, Oncopeptides, Roche/Genentech, Sanofi, and Takeda, served in a consulting or advisory role for GSK, received travel funding from Amgen, BMS, Celgene, GSK, Janssen, and Takeda, and received research funding from Amgen, Celgene, Janssen, and Sanofi.TM served in a consulting or advisory role for GlaxoSmithKline and Juno Therapeutics, and received research funding from Amgen, Janssen, and Sanofi.AK served in a consulting or advisory role for Adaptive Biotechnologies, Celgene/Bristol Meyers‐Squibb, GlaxoSmithKline, Janssen Oncology, Pfizer, Regeneron, served on speakers bureaus for Amgen, Celgene/Bristol Meyers‐Squibb, GlaxoSmithKline and Takeda, served on scientific advisory boards for Sutro Biopharma, has equity in Celgene/Bristol Meyers‐Squibb, and received research funding from Janssen Oncology.SJ is a consultant for Bristol Myers Squibb, Janssen, Karyopharm Therapeutics, Merck, Sanofi, and Takeda Pharmaceuticals.SZU served in a consulting or advisory role for AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Merck, Seattle Genetics, Skyline Diagnostics, and Takeda, served on speakers bureaus for Celgene, Janssen, Sanofi, and Takeda, and received research funding from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, and Skyline Diagnostics.JGB served in a consulting or advisory role for Bluebird Bio, Bristol Myers Squibb, Celgene, CRISPR Therapeutics, Janssen, Karyopharm Therapeutics, Kite/Gilead, Legend Biotech, Secura Bio, Servier, and Takeda, and received research funding from AbbVie, Acetylon Pharmaceuticals, Amgen, Bluebird Bio, Bristol Myers Squibb, Celgene, Celularity, Constellation Pharmaceuticals, CURIS, EMD Serono, Genentech/Roche, Glenmark, Ichnos Sciences, Janssen, Kesios Therapeutics, Lilly, Novartis, Poseida, Sanofi, Takeda, Teva, and Vivolux.KY is consultant physician and received honoraria from Janssen, GSK, Amgen Inc., Takeda, Sanofi and research funding from Janssen, Takeda, Sanofi.AL, CC, JMS, KQ, MV, AB, JD, SN, SV, and YO are employed by Janssen and have restricted stock units and/or stock options. CCJ is employed by Janssen and is a consultant physician at the Memorial Sloan Kettering Cancer Center. MM was employed by Janssen when the study was conducted.
Figures

Similar articles
-
Comparative Effectiveness of Ciltacabtagene Autoleucel in CARTITUDE-4 Versus Real-World Physician's Choice of Therapy from the Flatiron Registry in Lenalidomide-Refractory Multiple Myeloma.Adv Ther. 2025 Aug 6. doi: 10.1007/s12325-025-03308-2. Online ahead of print. Adv Ther. 2025. PMID: 40768190
-
Meta-analysis of ciltacabtagene autoleucel versus physician's choice therapy for the treatment of patients with relapsed or refractory multiple myeloma.Curr Med Res Opin. 2022 Oct;38(10):1759-1767. doi: 10.1080/03007995.2022.2100651. Epub 2022 Aug 10. Curr Med Res Opin. 2022. PMID: 35815818
-
Matching-adjusted indirect comparison of efficacy outcomes for ciltacabtagene autoleucel in CARTITUDE-1 versus idecabtagene vicleucel in KarMMa for the treatment of patients with relapsed or refractory multiple myeloma.Curr Med Res Opin. 2021 Oct;37(10):1779-1788. doi: 10.1080/03007995.2021.1953456. Epub 2021 Jul 23. Curr Med Res Opin. 2021. PMID: 34256668 Clinical Trial.
-
Cilta-cel, a BCMA-targeting CAR-T therapy for patients with multiple myeloma.Expert Opin Biol Ther. 2024 May;24(5):339-350. doi: 10.1080/14712598.2024.2352591. Epub 2024 May 13. Expert Opin Biol Ther. 2024. PMID: 38738379 Review.
-
Recent Advances in the Use of Chimeric Antigen Receptor-Expressing T-Cell Therapies for Treatment of Multiple Myeloma.Clin Lymphoma Myeloma Leuk. 2023 Jan;23(1):22-27. doi: 10.1016/j.clml.2022.09.001. Epub 2022 Sep 20. Clin Lymphoma Myeloma Leuk. 2023. PMID: 36411210 Review.
Cited by
-
CAR T-cell therapy for triple-class exposed relapsed/refractory multiple myeloma.Haematologica. 2023 Aug 1;108(8):1988-1990. doi: 10.3324/haematol.2022.282587. Haematologica. 2023. PMID: 37021536 Free PMC article. No abstract available.
-
Chimeric Antigen Receptor T-Cell and Bispecific Antibody Therapy in Multiple Myeloma: Moving Into the Future.J Clin Oncol. 2023 Sep 20;41(27):4416-4429. doi: 10.1200/JCO.23.00512. Epub 2023 Jul 20. J Clin Oncol. 2023. PMID: 37471687 Free PMC article. Review.
-
Teclistamab versus real-world physician's choice of therapy in triple-class exposed relapsed/refractory multiple myeloma.J Comp Eff Res. 2023 Jun;12(6):e220186. doi: 10.57264/cer-2022-0186. Epub 2023 Apr 28. J Comp Eff Res. 2023. PMID: 37114426 Free PMC article.
-
Efficacy of CARVYKTI in CARTITUDE-4 versus other conventional treatment regimens for lenalidomide-refractory multiple myeloma using inverse probability of treatment weighting.J Comp Eff Res. 2024 Sep;13(9):e240080. doi: 10.57264/cer-2024-0080. Epub 2024 Aug 20. J Comp Eff Res. 2024. PMID: 39162049 Free PMC article. Clinical Trial.
-
Comparative Effectiveness of Ciltacabtagene Autoleucel in CARTITUDE-4 Versus Real-World Physician's Choice of Therapy from the Flatiron Registry in Lenalidomide-Refractory Multiple Myeloma.Adv Ther. 2025 Aug 6. doi: 10.1007/s12325-025-03308-2. Online ahead of print. Adv Ther. 2025. PMID: 40768190
References
-
- Dimopoulos MA, Richardson PG, Moreau P, Anderson KC. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat Rev Clin Oncol. 2015;12(1):42–54. - PubMed
-
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk‐stratification and management. Am J Hematol. 2020;95(5):548–67. - PubMed
-
- Martin TG, Krishnan AY, Yong K, Weisel K, Mehra M, Nair S, et al. Comparison of outcomes with ciltacabtagene autoleucel (cilta‐cel) in CARTITUDE‐1 versus real‐world standard of care (RW SOC) for patients (pts) with triple‐class exposed relapsed/refractory multiple myeloma (RRMM). Abstract #8045. Poster presented at American Society of Clinical Oncology (ASCO) Annual Meeting; 2021. Jun 4–8; Virtual.
-
- Weisel KC, Martin T, Yong K, Qi K, Londhe A & Kobos R et al. Characteristics and treatment patterns of triple‐class exposed patients with relapsed/refractory multiple myeloma who participated in clinical trials of daratumumab. Oral presentation presented at 47th Annual Meeting of the European Society of Blood and Marrow Transplantation (EBMT); 2021 Mar 14–17.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
