Case Report: Inactivating PTH/PTHrP Signaling Disorder Type 1 Presenting With PTH Resistance
- PMID: 35846276
- PMCID: PMC9280615
- DOI: 10.3389/fendo.2022.928284
Case Report: Inactivating PTH/PTHrP Signaling Disorder Type 1 Presenting With PTH Resistance
Abstract
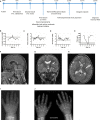
PTH resistance is characterized by elevated parathyroid hormone (PTH) levels, hypocalcemia, hyperphosphatemia and it is classically associated with GNAS locus genetic or epigenetic defects. Inactivating PTH/PTHrP signaling disorders (iPPSD) define overlapping phenotypes based on their molecular etiology. iPPSD1 is associated with PTH1R variants and variable phenotypes including ossification anomalies and primary failure of tooth eruption but no endocrine disorder. Here we report on a 10-month-old child born from consanguineous parents, who presented with mild neurodevelopmental delay, seizures, enlarged fontanelles, round face, and bilateral clinodactyly. Hand x-rays showed diffuse delayed bone age, osteopenia, short metacarpal bones and cone-shaped distal phalanges. A diagnosis of PTH resistance was made on the basis of severe hypocalcemia, hyperphosphatemia, elevated PTH and normal vitamin D levels on blood sample. The patient was treated with calcium carbonate and alfacalcidol leading to rapid bio-clinical improvement. Follow-up revealed multiple agenesis of primary teeth and delayed teeth eruption, as well as Arnold-Chiari type 1 malformation requiring a ventriculoperitoneal shunt placement. GNAS gene analysis showed no pathogenic variation, but a likely pathogenic homozygous substitution c.723C>G p.(Asp241Glu) in PTH1R gene was found by trio-based whole exome sequencing. We studied the deleterious impact of the variant on the protein conformation with bioinformatics tools. In conclusion, our study reports for the first time PTH resistance in a child with a biallelic PTH1R mutation, extending thereby the clinical spectrum of iPPSD1 phenotypes.
Keywords: Albright hereditary osteodystrophy; GNAS; PTH1R; alfacalcidol; epilepsy; iPPSD1; parathyroid hormone; pseudohypoparathyreoidism.
Copyright © 2022 Demaret, Wintjens, Sana, Docquir, Bertin, Ide, Monestier, Karadurmus, Benoit and Maystadt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ucciferro P, Anastasopoulou C. Pseudohypoparathyroidism. In: Statpearls. Treasure Island (FL): StatPearls Publishing; (2021).
-
- Sarathi V, Wadhwa R. Albright Hereditary Osteodystrophy. In: Statpearls. Treasure Island (FL): StatPearls Publishing; (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

