Receptor Guanylyl Cyclase C and Cyclic GMP in Health and Disease: Perspectives and Therapeutic Opportunities
- PMID: 35846281
- PMCID: PMC9276936
- DOI: 10.3389/fendo.2022.911459
Receptor Guanylyl Cyclase C and Cyclic GMP in Health and Disease: Perspectives and Therapeutic Opportunities
Abstract
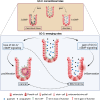
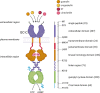
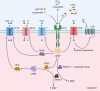
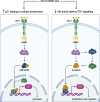
Receptor Guanylyl Cyclase C (GC-C) was initially characterized as an important regulator of intestinal fluid and ion homeostasis. Recent findings demonstrate that GC-C is also causally linked to intestinal inflammation, dysbiosis, and tumorigenesis. These advances have been fueled in part by identifying mutations or changes in gene expression in GC-C or its ligands, that disrupt the delicate balance of intracellular cGMP levels and are associated with a wide range of clinical phenotypes. In this review, we highlight aspects of the current knowledge of the GC-C signaling pathway in homeostasis and disease, emphasizing recent advances in the field. The review summarizes extra gastrointestinal functions for GC-C signaling, such as appetite control, energy expenditure, visceral nociception, and behavioral processes. Recent research has expanded the homeostatic role of GC-C and implicated it in regulating the ion-microbiome-immune axis, which acts as a mechanistic driver in inflammatory bowel disease. The development of transgenic and knockout mouse models allowed for in-depth studies of GC-C and its relationship to whole-animal physiology. A deeper understanding of the various aspects of GC-C biology and their relationships with pathologies such as inflammatory bowel disease, colorectal cancer, and obesity can be leveraged to devise novel therapeutics.
Keywords: cGMP (cyclic GMP); colorectal cancer type; guanylyl cyclase C; guanylyl cyclase C agonists; intestine.
Copyright © 2022 Prasad, Mathew and Visweswariah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21.J Biol Chem. 2014 Jan 3;289(1):581-93. doi: 10.1074/jbc.M113.511311. Epub 2013 Nov 11. J Biol Chem. 2014. PMID: 24217248 Free PMC article.
-
Guanylate Cyclase C: A Current Hot Target, from Physiology to Pathology.Curr Med Chem. 2018;25(16):1879-1908. doi: 10.2174/0929867325666171205150310. Curr Med Chem. 2018. PMID: 29210639 Review.
-
The evolutionary divergence of receptor guanylyl cyclase C has implications for preclinical models for receptor-directed therapeutics.J Biol Chem. 2024 Jan;300(1):105505. doi: 10.1016/j.jbc.2023.105505. Epub 2023 Nov 27. J Biol Chem. 2024. PMID: 38029963 Free PMC article.
-
The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis.FEBS Lett. 2012 Aug 31;586(18):2835-40. doi: 10.1016/j.febslet.2012.07.028. Epub 2012 Jul 20. FEBS Lett. 2012. PMID: 22819815 Review.
-
Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases.Pharmacol Ther. 2011 Apr;130(1):71-82. doi: 10.1016/j.pharmthera.2010.12.005. Epub 2010 Dec 24. Pharmacol Ther. 2011. PMID: 21185863 Free PMC article. Review.
Cited by
-
Microbiota-derived butyrate dampens linaclotide stimulation of the guanylate cyclase C pathway in patient-derived colonoids.Neurogastroenterol Motil. 2023 Dec;35(12):e14681. doi: 10.1111/nmo.14681. Epub 2023 Sep 22. Neurogastroenterol Motil. 2023. PMID: 37736865 Free PMC article.
-
Transcriptomic Maps of Colorectal Liver Metastasis: Machine Learning of Gene Activation Patterns and Epigenetic Trajectories in Support of Precision Medicine.Cancers (Basel). 2023 Jul 28;15(15):3835. doi: 10.3390/cancers15153835. Cancers (Basel). 2023. PMID: 37568651 Free PMC article.
-
Association of Uroguanylin, Body Mass Index, and Waist Circumference: Sex Differences and Obesity Implications among a Sample of Iraqi Adults in Baghdad City.J Pharm Bioallied Sci. 2024 Feb;16(Suppl 1):S406-S408. doi: 10.4103/jpbs.jpbs_632_23. Epub 2024 Feb 29. J Pharm Bioallied Sci. 2024. PMID: 38595491 Free PMC article.
-
Navigating the future of gastric cancer treatment: a review on the impact of antibody-drug conjugates.Cell Death Discov. 2025 Apr 5;11(1):144. doi: 10.1038/s41420-025-02429-5. Cell Death Discov. 2025. PMID: 40188055 Free PMC article. Review.
-
Breaking Down Barriers: Epithelial Contributors to Monogenic IBD Pathogenesis.Inflamm Bowel Dis. 2024 Jul 3;30(7):1189-1206. doi: 10.1093/ibd/izad319. Inflamm Bowel Dis. 2024. PMID: 38280053 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

