Immuno-Modulatory Effects of Intervertebral Disc Cells
- PMID: 35846355
- PMCID: PMC9277224
- DOI: 10.3389/fcell.2022.924692
Immuno-Modulatory Effects of Intervertebral Disc Cells
Abstract
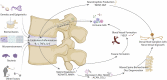
Low back pain is a highly prevalent, chronic, and costly medical condition predominantly triggered by intervertebral disc degeneration (IDD). IDD is often caused by structural and biochemical changes in intervertebral discs (IVD) that prompt a pathologic shift from an anabolic to catabolic state, affecting extracellular matrix (ECM) production, enzyme generation, cytokine and chemokine production, neurotrophic and angiogenic factor production. The IVD is an immune-privileged organ. However, during degeneration immune cells and inflammatory factors can infiltrate through defects in the cartilage endplate and annulus fibrosus fissures, further accelerating the catabolic environment. Remarkably, though, catabolic ECM disruption also occurs in the absence of immune cell infiltration, largely due to native disc cell production of catabolic enzymes and cytokines. An unbalanced metabolism could be induced by many different factors, including a harsh microenvironment, biomechanical cues, genetics, and infection. The complex, multifactorial nature of IDD brings the challenge of identifying key factors which initiate the degenerative cascade, eventually leading to back pain. These factors are often investigated through methods including animal models, 3D cell culture, bioreactors, and computational models. However, the crosstalk between the IVD, immune system, and shifted metabolism is frequently misconstrued, often with the assumption that the presence of cytokines and chemokines is synonymous to inflammation or an immune response, which is not true for the intact disc. Therefore, this review will tackle immunomodulatory and IVD cell roles in IDD, clarifying the differences between cellular involvements and implications for therapeutic development and assessing models used to explore inflammatory or catabolic IVD environments.
Keywords: GWAS; agent-based model (ABM); artificial intelligence–AI; catabolism; immune-privileged microenvironment; inflammation; intervertebral disc degeneration; low back pain.
Copyright © 2022 Bermudez-Lekerika, Crump, Tseranidou, Nüesch, Kanelis, Alminnawi, Baumgartner, Muñoz-Moya, Compte, Gualdi, Alexopoulos, Geris, Wuertz-Kozak, Le Maitre, Noailly and Gantenbein.
Conflict of interest statement
EK and LK were employed by ProtATonce Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction.Spine J. 2005 Jan-Feb;5(1):14-23. doi: 10.1016/j.spinee.2004.05.251. Spine J. 2005. PMID: 15653081
-
Current Knowledge and Future Therapeutic Prospects in Symptomatic Intervertebral Disc Degeneration.Yonsei Med J. 2022 Mar;63(3):199-210. doi: 10.3349/ymj.2022.63.3.199. Yonsei Med J. 2022. PMID: 35184422 Free PMC article. Review.
-
Intervertebral Disc Degeneration Models for Pathophysiology and Regenerative Therapy -Benefits and Limitations.J Invest Surg. 2022 Apr;35(4):935-952. doi: 10.1080/08941939.2021.1953640. Epub 2021 Jul 26. J Invest Surg. 2022. PMID: 34309468 Review.
-
Role of cytokines in intervertebral disc degeneration: pain and disc content.Nat Rev Rheumatol. 2014 Jan;10(1):44-56. doi: 10.1038/nrrheum.2013.160. Epub 2013 Oct 29. Nat Rev Rheumatol. 2014. PMID: 24166242 Free PMC article. Review.
-
Gut-disc axis: A cause of intervertebral disc degeneration and low back pain?Eur Spine J. 2022 Apr;31(4):917-925. doi: 10.1007/s00586-022-07152-8. Epub 2022 Mar 14. Eur Spine J. 2022. PMID: 35286474 Review.
Cited by
-
The role of miR-155-5p in inflammation and mechanical loading during intervertebral disc degeneration.Cell Commun Signal. 2024 Aug 28;22(1):419. doi: 10.1186/s12964-024-01803-7. Cell Commun Signal. 2024. PMID: 39192354 Free PMC article.
-
Cartilaginous endplates: A comprehensive review on a neglected structure in intervertebral disc research.JOR Spine. 2023 Oct 21;6(4):e1294. doi: 10.1002/jsp2.1294. eCollection 2023 Dec. JOR Spine. 2023. PMID: 38156054 Free PMC article. Review.
-
Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration.Int J Mol Sci. 2022 Dec 22;24(1):208. doi: 10.3390/ijms24010208. Int J Mol Sci. 2022. PMID: 36613651 Free PMC article. Review.
-
Experimental validation and comprehensive analysis of m6A methylation regulators in intervertebral disc degeneration subpopulation classification.Sci Rep. 2024 Apr 10;14(1):8417. doi: 10.1038/s41598-024-58888-w. Sci Rep. 2024. PMID: 38600232 Free PMC article.
-
YTHDF2-dependent m6A modification of FOXO3 mRNA mediates TIMP1 expression and contributes to intervertebral disc degeneration following ROS stimulation.Cell Mol Life Sci. 2024 Dec 3;81(1):477. doi: 10.1007/s00018-024-05503-w. Cell Mol Life Sci. 2024. PMID: 39625652 Free PMC article.

