Single-shot AAV-vectored vaccine against SARS-CoV-2 with fast and long-lasting immunity
- PMID: 35846427
- PMCID: PMC9273293
- DOI: 10.1016/j.apsb.2022.07.004
Single-shot AAV-vectored vaccine against SARS-CoV-2 with fast and long-lasting immunity
Abstract
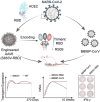
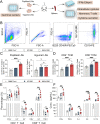
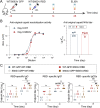
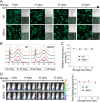
Due to the insufficient long-term protection and significant efficacy reduction to new variants of current COVID-19 vaccines, the epidemic prevention and control are still challenging. Here, we employ a capsid and antigen structure engineering (CASE) strategy to manufacture an adeno-associated viral serotype 6-based vaccine (S663V-RBD), which expresses trimeric receptor binding domain (RBD) of spike protein fused with a biological adjuvant RS09. Impressively, the engineered S663V-RBD could rapidly induce a satisfactory RBD-specific IgG titer within 2 weeks and maintain the titer for more than 4 months. Compared to the licensed BBIBP-CorV (Sinopharm, China), a single-dose S663V-RBD induced more endurable and robust immune responses in mice and elicited superior neutralizing antibodies against three typical SARS-CoV-2 pseudoviruses including wild type, C.37 (Lambda) and B.1.617.2 (Delta). More interestingly, the intramuscular injection of S663V-RBD could overcome pre-existing immunity against the capsid. Given its effectiveness, the CASE-based S663V-RBD may provide a new solution for the current and next pandemic.
Keywords: AAV vectors; Antigen structure design; Capsid engineering; SARS-CoV-2; Vaccine.
© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures




References
-
- WHO Coronavirus (COVID-19) Dashboard. Availabe from: https://covid19.who.int/.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

