Safety Profile and Tolerability of Topical Phosphodiesterase 4 Inhibitors for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis
- PMID: 35846836
- PMCID: PMC9278032
- DOI: 10.1016/j.curtheres.2022.100679
Safety Profile and Tolerability of Topical Phosphodiesterase 4 Inhibitors for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis
Abstract
Objective: Evaluate the safety profile and tolerability of topical phosphodiesterase 4 (PDE4) inhibitors versus vehicle as treatment for atopic dermatitis in published studies.
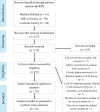
Methods: A search was performed in Medline/PubMed, Web of Science, and Cochrane Library databases on September 27, 2021, by 1 evaluator, without restrictions on publication dates or languages. Terms such as atopic dermatitis, phosphodiesterase 4 inhibitors, calcineurin inhibitors, and randomized controlled trials were included. The database searches were carried out by 1 evaluator. The titles and abstracts were reviewed for the identification and evaluation of potentially eligible studies. Study selection was made by two reviewers, so there was no intra-examiner statistic at the study selection step. The full-text articles were reviewed to determine whether or not they would be included in the systematic review. Global analyses, which included studies with both unclear and low risk of bias and subanalyses of studies with a low risk of bias were performed.
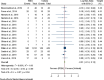
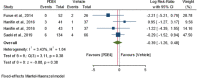
Results: Out of 237 identified articles, 14 clinical trials were included in the meta-analysis. In global analyses of studies with low and unclear risk of bias, topical treatment with PDE4 inhibitors did not differ from vehicle treatment in global treatment emergent adverse events (relative risk = 0.99; 95% CI, 0.87-1.14; P = 0.94) or in serious emergent adverse events appearance (relative risk = 0.92; 95% CI, 0.39-2.20; P = 0.86). In subanalyses of studies with a low risk of bias, a reduced rate of atopic dermatitis exacerbation was observed in PDE4 inhibitors compared with the vehicle (relative risk = 0.62; 95% CI, 0.39-0.98; P = 0.04) and risk of pain at the application site was confirmed (relative risk = 2.59; 95% CI, 1.27-5.28; P = 0.01).
Conclusions: PDE4 inhibitors did not show differences from vehicle treatment in treatment emergent adverse events or serious emergent adverse events incidence. In studies with low risk of bias, PDE4 inhibitors had a statistically significant risk of producing pain and reduced occurrence of atopic dermatitis exacerbation.
Keywords: Atopic dermatitis; Crisaborole; Meta-analysis; Phosphodiesterase 4 inhibitors; Topical treatment.
© 2022 The Author(s).
Figures



References
-
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–360. - PubMed
-
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66 Suppl 1:8–16. - PubMed
-
- Bissonnette R, Pavel AB, Diaz A, et al. Crisaborole and atopic dermatitis skin biomarkers: An intrapatient randomized trial. J Allergy Clin Immunol. 2019;144:1274–1289. - PubMed
-
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol. 2017;139:1723–1734. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

