Engineering microparticles based on solidified stem cell secretome with an augmented pro-angiogenic factor portfolio for therapeutic angiogenesis
- PMID: 35846945
- PMCID: PMC9270501
- DOI: 10.1016/j.bioactmat.2022.03.015
Engineering microparticles based on solidified stem cell secretome with an augmented pro-angiogenic factor portfolio for therapeutic angiogenesis
Abstract
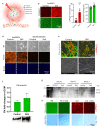
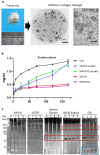
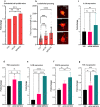
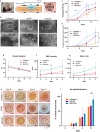
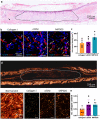
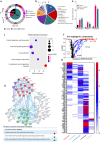
Tissue (re)vascularization strategies face various challenges, as therapeutic cells do not survive long enough in situ, while the administration of pro-angiogenic factors is hampered by fast clearance and insufficient ability to emulate complex spatiotemporal signaling. Here, we propose to address these limitations by engineering a functional biomaterial capable of capturing and concentrating the pro-angiogenic activities of mesenchymal stem cells (MSCs). In particular, dextran sulfate, a high molecular weight sulfated glucose polymer, supplemented to MSC cultures, interacts with MSC-derived extracellular matrix (ECM) components and facilitates their co-assembly and accumulation in the pericellular space. Upon decellularization, the resulting dextran sulfate-ECM hybrid material can be processed into MIcroparticles of SOlidified Secretome (MIPSOS). The insoluble format of MIPSOS protects protein components from degradation, while facilitating their sustained release. Proteomic analysis demonstrates that MIPSOS are highly enriched in pro-angiogenic factors, resulting in an enhanced pro-angiogenic bioactivity when compared to naïve MSC-derived ECM (cECM). Consequently, intravital microscopy of full-thickness skin wounds treated with MIPSOS demonstrates accelerated revascularization and healing, far superior to the therapeutic potential of cECM. Hence, the microparticle-based solidified stem cell secretome provides a promising platform to address major limitations of current therapeutic angiogenesis approaches.
Keywords: Dextran sulfate; Extracellular matrix; Mesenchymal stem cells; Poly-electrolyte-driven co-assembly; Therapeutic angiogenesis; Wound healing.
© 2022 The Authors.
Conflict of interest statement
AB and MA would like to declare the filing of a PCT patent application (WO 2020/228733 A1).
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

