Vancomycin Containing PDLLA and PLGA/β-TCP Inhibit Biofilm Formation but Do Not Stimulate Osteogenic Transformation of Human Mesenchymal Stem Cells
- PMID: 35846965
- PMCID: PMC9283789
- DOI: 10.3389/fsurg.2022.885241
Vancomycin Containing PDLLA and PLGA/β-TCP Inhibit Biofilm Formation but Do Not Stimulate Osteogenic Transformation of Human Mesenchymal Stem Cells
Abstract
Aims: Chronic osteomyelitis, including implant-related prosthetic joint infection, is extremely difficult to cure. We develop vancomycin containing release systems from poly(d,l-lactide) (PDLLA) and poly(d,l-lactide-co-glycolide) (PLGA) composites with beta-tricalcium phosphate (β-TCP) to treat methicillin-resistant Staphylococcus aureus osteomyelitis. We ask whether vancomycin containing PDLLA/β-TCP and PLGA/β-TCP composites will prevent early biofilm formation, allow cell proliferation and osteogenic differentiation, and stimulate osteogenic signaling molecules in the absence of an osteogenic medium.
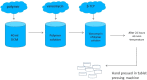
Methods: Composites were produced and characterized with scanning electron microscopy. In vitro vancomycin release was assessed for 6 weeks. Biofilm prevention was calculated by crystal violet staining. Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) and osteosarcoma cell (SaOS-2) proliferation and differentiation were assessed with water soluble tetrazolium salt and alkaline phosphatase (ALP) staining. Real-time quantitative polymerase chain reaction defined osteogenic signaling molecules for hBM-MSCs.
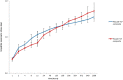
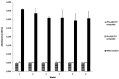
Results: Totally, 3.1 ± 0.2 mg and 3.4 ± 0.4 mg vancomycin released from PDLLA/β-TCP and the PLGA/β-TCP composites, respectively, and inhibited early biofilm formation. hBM-MSCs and SaOS-2 cells proliferated on the composites and stimulated ALP activity of cells. Runt-related transcription factor 2 (RUNX2) and SRY-Box transcription Factor 9 (SOX9) expressions were, however, lower with composites when compared with control.
Conclusion: Vancomycin containing PDLLA/β-TCP and PLGA/β-TCP composites inhibited early biofilm formation and proliferated and differentiated hBM-MSCs and SaOS-2 cells, but osteogenesis-related RUNX2 and SOX9 transcription factors were not strongly expressed in the absence of an osteogenic medium for 14 days.
Keywords: PDLLA; PLGA; biofilm; bone signaling molecules; vancomycin; β-TCP.
Copyright © 2022 Kankilic, Bayramli, Korkusuz, Eroglu, Sener, Mutlu and Korkusuz.
Conflict of interest statement
One or more of the authors (BK, EB, PK, and FK) has received funding from the Republic of Turkey Ministry of Science, Industry and Technology SANTEZ Programme Project No: 00817.STZ.2011-1.
Figures


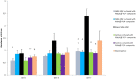
References
LinkOut - more resources
Full Text Sources
Research Materials

