Relation Between Gender and Concomitant Medications With Erythropoietin-Treatment on Wound Healing in Burn Patients. Post Hoc Subgroup-Analysis of the Randomized, Placebo-Controlled Clinical Trial "EPO in Burns"
- PMID: 35847006
- PMCID: PMC9284535
- DOI: 10.3389/fphar.2022.812888
Relation Between Gender and Concomitant Medications With Erythropoietin-Treatment on Wound Healing in Burn Patients. Post Hoc Subgroup-Analysis of the Randomized, Placebo-Controlled Clinical Trial "EPO in Burns"
Abstract
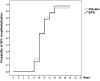
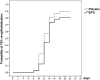
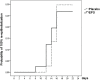
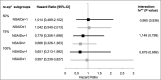
Burns are leading causes of mortality and morbidity, including prolonged hospitalization, disfigurement, and disability. Erythropoietin (EPO) is a well-known hormone causing erythropoiesis. However, EPO may play a role in healing acute and chronic wounds due to its anti-inflammatory and pro-regenerative effects. Therefore, the large, prospective, placebo-controlled, randomized, double-blind, multi-center clinical trial "EPO in Burns" was initiated to investigate the effects of EPO versus placebo treatment in severely burned patients. The primary endpoint of "EPO in Burns" was defined as the time elapsed until complete re-epithelialization of a defined split skin graft donor site. Additional analyses of post hoc defined subgroups were performed in view of the primary endpoint. The verum (n 45) and control (n 39) groups were compared with regard to the time it took for study wounds (a predefined split skin graft donor site) to reach the three stages of wound healing (re-epithelialization levels). In addition, the effects of gender (females n 18) and concomitant medications insulin (n 36), non-steroidal anti-inflammatory drugs (NSAIDs) (n 41), and vasopressor agents (n 43) were tested. Life tables were used to compare study groups (EPO vs. placebo) within subgroups. The Cox regression model was applied to evaluate interactions between the study drug (EPO) and concomitant medications for each re-epithelialization level. Using our post hoc defined subgroups, we observed a lower chance of wound healing for women compared to men (in terms of hazard ratio: hr100%: 5.984 [95%-CI: (0.805-44.490), p = 0.080]) in our study population, regardless of the study medication. In addition, results indicated an earlier onset of re-epithelialization in the first days of EPO treatment (EPO: 10% vs. Placebo: 3%). Moreover, the interpretation of the hazard ratio suggested EPO might have a positive, synergistic effect on early stages of re-epithelialization when combined with insulin [hr50%: 1.307 (p = 0.568); hr75%: 1,199 (p = 0.715)], as well as a stabilizing effect on critically ill patients [reduced need for vasopressors in the EPO group (EPO: 44% vs. Placebo 59%)]. However, additional high-quality data from clinical trials designed to address these endpoints are required to gain further insight into these effects.
Keywords: burn injuries; erythropoietin (EPO); gender; randomized clinical trial; regenerative medicine; wound healing.
Copyright © 2022 Günter, Ilg, Hapfelmeier, Egert-Schwender, Jelkmann, Giri, Bader, Machens and EPO in Burns Study Group.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A Randomized Controlled Trial: Regenerative Effects, Efficacy and Safety of Erythropoietin in Burn and Scalding Injuries.Front Pharmacol. 2018 Oct 31;9:951. doi: 10.3389/fphar.2018.00951. eCollection 2018. Front Pharmacol. 2018. PMID: 30429786 Free PMC article.
-
A multi-center study on the regenerative effects of erythropoietin in burn and scalding injuries: study protocol for a randomized controlled trial.Trials. 2013 May 3;14:124. doi: 10.1186/1745-6215-14-124. Trials. 2013. PMID: 23782555 Free PMC article. Clinical Trial.
-
Effects of human recombinant growth hormone on donor-site healing in burned adults.World J Surg. 2002 Jan;26(1):2-8. doi: 10.1007/s00268-001-0170-9. Epub 2001 Oct 25. World J Surg. 2002. PMID: 11898025 Clinical Trial.
-
A review of negative-pressure wound therapy in the management of burn wounds.Burns. 2016 Dec;42(8):1623-1633. doi: 10.1016/j.burns.2016.06.011. Epub 2016 Jul 1. Burns. 2016. PMID: 27378361 Review.
-
[Erythropoietin in plastic surgery].Handchir Mikrochir Plast Chir. 2013 Apr;45(2):108-19. doi: 10.1055/s-0033-1334909. Epub 2013 Apr 29. Handchir Mikrochir Plast Chir. 2013. PMID: 23629685 Review. German.
Cited by
-
Frailty as a sequela of burn injury: a post hoc analysis of the "RE-ENERGIZE" multicenter randomized-controlled trial and the National Health Interview Survey.Mil Med Res. 2024 Sep 12;11(1):63. doi: 10.1186/s40779-024-00568-x. Mil Med Res. 2024. PMID: 39267196 Free PMC article. Clinical Trial.
References
-
- Alural B., Duran G. A., Tufekci K. U., Allmer J., Onkal Z., Tunali D., et al. (2014). EPO Mediates Neurotrophic, Neuroprotective, Anti-Oxidant, and Anti-Apoptotic Effects via Downregulation of miR-451 and miR-885-5p in SH-Sy5y Neuron-Like Cells. Front. Immunol. 5, 475. 10.3389/fimmu.2014.00475 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

