Cost-Effectiveness of Apatinib and Cabozantinib for the Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer
- PMID: 35847009
- PMCID: PMC9280160
- DOI: 10.3389/fphar.2022.860615
Cost-Effectiveness of Apatinib and Cabozantinib for the Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer
Abstract
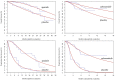
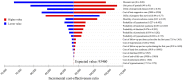
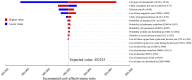
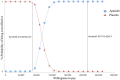
Background: The effectiveness of apatinib and cabozantinib for the treatment of radioactive iodine-refractory differentiated thyroid cancer (RAIR-DTC) has been demonstrated recently. We aimed to evaluate the cost-effectiveness of these treatments from the Chinese healthcare system perspective. Methods: Two partitioned survival models over a 10-year horizon were built to compare the cost and effectiveness of apatinib vs. placebo and cabozantinib vs. placebo based on the clinical data from the phase 3 randomized REALITY and COSMIC-311 trials. Costs and utility data were obtained from the literature and institutional database. The incremental cost-effectiveness ratio (ICER) was calculated. One-way and probabilistic sensitivity analysis was performed to test the robustness of the conclusion. Results: Apatinib yielded an additional quality-adjusted life-year (QALY) of 0.74 at an additional cost of Chinese Renminbi ¥44,077. The ICER was ¥93,460 (US dollar $13545)/QALY and it was below the current willingness-to-pay (WTP) threshold of ¥217341/QALY. Cabozantinib was associated with an additional QALY of 0.79 at an extra cost of ¥3,55,614 when compared with placebo, and the ICER was ¥4,52,325 ($65,554)/QALY, which was above the WTP threshold. The conclusion were robust under one-way and probabilistic sensitivity analysis. The price of cabozantinib has to drop to ¥5.87/mg (39% of the current price) for it has a 50% likelihood of being cost-effective. Conclusion: Apatinib is cost-effective for RAIR-DTC when compared with placebo from the perspective of Chinese healthcare system. However, based on the current evidence, cabozantinib might not be cost-effective and a reduction of price is warranted.
Keywords: China; apatinib; cabozantinib; cost-effective; radioactive iodine-refractory differentiated thyroid cancer.
Copyright © 2022 Shi, Ma, Pan, Shi, Zhang and Xing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cost-effectiveness analysis of cabozantinib as second-line therapy in advanced hepatocellular carcinoma.Liver Int. 2019 Dec;39(12):2408-2416. doi: 10.1111/liv.14257. Epub 2019 Oct 3. Liver Int. 2019. PMID: 31544330
-
Economic Evaluation of Sacituzumab Govitecan for the Treatment of Metastatic Triple-Negative Breast Cancer in China and the US.Front Oncol. 2021 Oct 28;11:734594. doi: 10.3389/fonc.2021.734594. eCollection 2021. Front Oncol. 2021. PMID: 34778047 Free PMC article.
-
Cost Effectiveness of Lenvatinib, Sorafenib and Placebo in Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer.Thyroid. 2017 Aug;27(8):1043-1052. doi: 10.1089/thy.2016.0572. Epub 2017 Jun 7. Thyroid. 2017. PMID: 28486081
-
Multi-gene Pharmacogenomic Testing That Includes Decision-Support Tools to Guide Medication Selection for Major Depression: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Aug 12;21(13):1-214. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34484487 Free PMC article.
-
Azacitidine for Treating Acute Myeloid Leukaemia with More Than 30 % Bone Marrow Blasts: An Evidence Review Group Perspective of a National Institute for Health and Care Excellence Single Technology Appraisal.Pharmacoeconomics. 2017 Mar;35(3):363-373. doi: 10.1007/s40273-016-0453-5. Pharmacoeconomics. 2017. PMID: 27752999 Review.
References
-
- Baio G. (2020). survHE: Survival Analysis for Health Economic Evaluation and Cost-Effectiveness Modeling. J. Stat. Softw. 95 (1), 1–47. 10.18637/jss.v095.i14 - DOI
-
- Brose M. S., Nutting C. M., Jarzab B., Elisei R., Siena S., Bastholt L., et al. (2014). Sorafenib in Radioactive Iodine-Refractory, Locally Advanced or Metastatic Differentiated Thyroid Cancer: a Randomised, Double-Blind, Phase 3 Trial. Lancet 384 (9940), 319–328. 10.1016/S0140-6736(14)60421-9 - DOI - PMC - PubMed
-
- Brose M. S., Robinson B., Sherman S. I., Krajewska J., Lin C. C., Vaisman F., et al. (2021). Cabozantinib for Radioiodine-Refractory Differentiated Thyroid Cancer (COSMIC-311): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 22 (8), 1126–1138. 10.1016/S1470-2045(21)00332-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources

